Discussion Paper on the Effects of Arsenic in the Maternal Diet
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Background
1. The Scientific Advisory Committee on Nutrition (SACN) last considered maternal diet and nutrition concerning offspring health in its reports on ‘The influence of maternal, fetal and child nutrition on the development of chronic disease in later life’ (SACN, 2011) and on ‘Feeding in the first year of life’ (SACN, 2018). In the latter report, the impact of breastfeeding on maternal health was also considered.
2. In 2019, SACN agreed to conduct a risk assessment on nutrition and maternal health focusing on maternal outcomes during pregnancy, childbirth, and up to 24 months after delivery; this would include the effects of chemical contaminants and excess nutrients in the diet.
3. SACN agreed that, where appropriate, other expert Committees would be consulted and asked to complete relevant risk assessments e.g., in the area of food safety advice. This subject was initially discussed during the horizon scanning item at the January 2020 meeting with a scoping paper being presented to the Committee in July 2020. This included background information on a provisional list of chemicals proposed by SACN. It was noted that the provisional list of chemicals was subject to change following discussion by the Committee on Toxicity of Chemicals in Food, Consumer Products, and the Environment (COT) which would be guiding the toxicological risk assessment process: candidate chemicals or chemical classes can be added or removed as the COT considered appropriate. The list was brought back to the COT with additional information in September 2020. Following discussion at the meeting, it was agreed that papers on several components should be prioritised and to this end, papers on iodine, vitamin D, and dietary supplements have been or will be presented to the Committee. The remaining list of compounds was to be triaged based on toxicity and exposure.
4. Following the discussion of the first prioritisation paper on substances to be considered for risk assessment by the COT, the Committee decided that each of the heavy metals (lead, mercury, cadmium, and arsenic) should be considered in separate papers. The following paper discusses the risks posed to maternal health by arsenic in the diet and the environment.
5. There is currently little to no advice for pregnant women and women of gestational age relating to arsenic. The United Kingdom (UK) Government (2022) website briefly mentions several epidemiology studies surrounding elevated arsenic consumption and the corresponding outcomes. However, the website does not provide advice to be followed (Public Health England, 2016a; GOV.UK, 2019). There is only minimal advice provided by the National Health Service (NHS) relating to arsenic exposure for adults, but the U.S. Food and Drug Administration (FDA) provides ‘tips’ to limit exposure including testing well water sources, eating a varied nutritious diet, and understanding juice and rice consumption for children (FDA, 2022).
6. The current governmental dietary advice for infants and young children relating to arsenic is that children under 5 years old should not be given rice drinks as a substitute for breast milk, infant formula, or cow’s milk. This is due to the potential for rice drinks to contain high levels of arsenic, and this age group’s proportionally higher milk consumption and lower body weights compared to other consumers (FSA, 2018). In addition, the NHS advises that cows’ milk or alternatives are not suitable as drinks for infants under 12 months old. The advice regarding rice drink consumption provided on the NHS Safe Weaning page is precautionary and states that children under five years of age shouldn’t consume rice drinks as they contain high levels of arsenic (NHS, 2022).
7. On 25th June 2015, the European Commission (EC) set maximum levels (MLs) for inorganic arsenic (iAs) in rice and rice-based products; these MLs are presented in Table 1. The European Food Safety Authority (EFSA) scientific opinion (EFSA, 2009) identified that high consumers within specific groups, e.g., certain ethnic groups and children under three, are most exposed to inorganic dietary arsenic. It was also found that dietary exposure to iAs for children under three years old is estimated to be 2- to 3-fold that of adults. The EC stated that the MLs were set specifically for rice and rice-based products because the analysis of iAs in these foods is reliable. Different MLs were proposed given the differing arsenic contents of these foods (Commission Regulation (EU) 2015/1006) (EC, 2015).
Table 1. Maximum levels of iAs permitted in rice and rice-based products (Commission Regulation (EU) 2015/1006).
|
Food Group |
Maximum Level (µg/kg) |
|
Non-parboiled milled rice (polished or white rice) |
200 |
|
Parboiled rice and husked rice |
250 |
|
Rice waffles, rice wafers, rice crackers, and rice cakes |
300 |
|
Rice destined for the production of foods for infants and young children* |
100 |
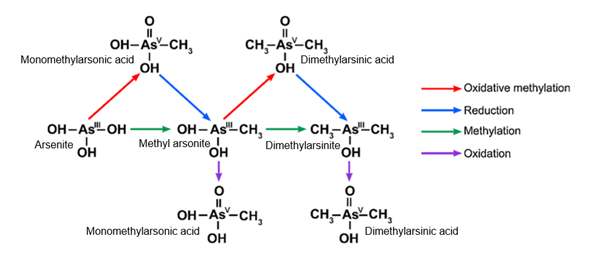
* Foodstuffs listed in this category as defined in Commission Directive 2006/125/EC of 5th December 2006.
Introduction
8. The Royal Society of Chemistry (RSC) describes arsenic (As) as a bright, silvery-grey group 15 metal, with an atomic number of 33 and a relative atomic mass of 75. Arsenic is used as a poison and insecticide, feed additive to prevent disease and improve weight gain in poultry, and in engineering, e.g. as a doping agent in semiconductors such as gallium arsenide (The Royal Society of Chemistry, 2023).
9. Arsenic is a metalloid that occurs in the environment in a variety of forms as a result of both natural and anthropogenic activity. It is generally accepted that iAs compounds are more toxic than the organic As compounds that are commonly found in fish, seafood, and other marine organisms (arsenobetaine (AB), arsenosugars, and arsenolipids). The iAs present in the environment contains many species of As, primarily in the trivalent or pentavalent oxidation states. These species are comprised mainly of complexes, such as dimethylmonoarsenate (DMA), or as arsenite (As(III)) and arsenate (As(V)) oxoanions in the +3 and +5 oxidation states respectively. In food samples, iAs is often reported as As(III) and As(V), or as the sum of these as total arsenic (tAs), even though it is likely bound to peptides or proteins in the food itself (EFSA CONTAM, 2009). The key As species discussed in this paper are summarised in Annex 1.
Previous Evaluations
10. Expert opinions on exposure to As in food have been published by EFSA’s Panel on Contaminants in the Food Chain (CONTAM) (EFSA CONTAM, 2009) and the Joint Food and Agriculture Organization (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) (FAO/WHO, 2011). The World Health Organization has also reviewed exposures to As via drinking water as part of the development of their ‘Guidelines for Drinking Water Quality’ (WHO, 2022). The International Agency for Research on Cancer (IARC) has published an evaluation of the carcinogenicity of As and As compounds (IARC, 2018), and the COT published opinions on As in the diet of infants and young children in 2016 and in response to the total dietary survey in 1999 (COT, 2003, 2016).
11. In 1983, JECFA established an association between exposure of humans to iAs from drinking water and increased cancer risk. A concentration of 0.2 mg iAs/L was associated with a 5% increase in the lifetime risk of skin cancer from epidemiological evidence. The available epidemiological evidence allowed the tentative conclusion that iAs could be present in water supplies containing an upper iAs concentration of 1 mg/L or greater, and that a concentration of 0.1 mg/L may give rise to presumptive signs of toxicity. The chemical species of As present in the drinking water were not clearly determined but JECFA concluded it was reasonable to consider them to be iAs. Assuming a daily water consumption of 1.5 litres, JECFA concluded that intakes of 1.5 mg/day of iAs were likely to result in chronic As toxicity and daily intakes of 0.15 mg may also be toxic in the long term to some individuals. JECFA set a Provisional Maximum Tolerable Daily Intake (PMTDI) of 2 µg/kg bw/day, however, the rationale for the PMTDI was unclear (FAO/WHO, 1993).
12. In 1989 JECFA confirmed their previous evaluation of iAs by assigning it a provisional tolerable weekly intake (PTWI) of 15 μg/kg bw based on the previous PMTDI but stated that “the margin between the PTWI and intakes reported to have toxic effects in epidemiological studies was narrow” (FAO/WHO, 1989). Both the COT in 2008 and EFSA in 2009 concluded that this PTWI was not appropriate and stated that the approach used to establish the PTWI could no longer be considered suitable, given the evidence of genotoxicity and carcinogenicity. Data have shown that iAs causes cancers of the lung, urinary bladder and skin. (COT, 2008; EFSA, 2009) .
13. By modelling the available dose-response data from key epidemiological studies, and selecting a benchmark response of 1% extra risk, the EFSA CONTAM Panel (2009) established a range of values for the 95% lower confidence limit of the benchmark dose (BMDL01) of 0.3 to 8 μg/kg bw/day for iAs. This range of BMDL01 values was identified for cancers of the lung, skin, and bladder, as well as skin lesions. EFSA decided that a range should be used instead of a single reference point for the risk characterisation of iAs. The CONTAM panel concluded that iAs was not directly DNA-reactive and there are numerous proposed mechanisms for carcinogenicity. With this, and the uncertainty surrounding the shape of the dose-response relationships, an assessment was made using a margin of exposure (MOE) approach. As the estimated exposures fell within the range of BMDL01 values for average and high-level consumers in Europe, EFSA concluded there was little or no margin of exposure and that the possibility of a risk to some consumers could not be excluded.
14. Overall, the EFSA CONTAM Panel (2009) recommended that dietary exposure to iAs should be reduced. To refine the risk assessment for iAs, the Panel recommended that speciation data be produced for different food commodities to support dietary exposure assessment and dose-response data for the possible health effects (EFSA CONTAM, 2009).
15. The EFSA (2009) paper also described a study where a cohort of 10-year-olds showed impaired intellectual function and another study showing a negative association between increasing tAs exposure and intellectual function in 6-year olds. However, these findings were contradicted by a study of children aged between 5 to 15 years following tAs exposure via drinking water. No evidence was found to associate increased iAs concentrations in urine and neurological test results during pregnancy and early childhood (EFSA CONTAM, 2009).
16. Prior review (COT, 2016) has shown few data are available regarding the toxicity of organic As compounds in humans and exposure to such compounds is not generally considered to be of toxicological concern. Arsenic toxicity studies have mainly focussed on AB, the main organoarsenical found in seafood, and the general assumption of non-toxicity of the organic compounds. In more recent years, progress has been shown regarding metabolism and toxicity studies for other organoarsenicals. Re-evaluation of organic As has started due to the discovery of extreme toxicity of trivalent methyl arsenicals and the cytotoxicity shown by As-containing hydrocarbons to human liver and bladder cells (Xue et al., 2021). The new studies pertaining to the potential toxicity of organic As species are discussed in the toxicity section of this paper.
17. In place of the PTWI, JECFA in 2011 determined a single BMDL for a 0.5% increased incidence of lung cancer above background; the value calculated for the 95% lower confidence limit of the BMDL0.5 was 3.0 µg/kg bw/day. This was the lowest of the BMDLs calculated by the JECFA. Sensitivity analysis, undertaken to investigate the impact of uncertainty in the exposure estimates in the study upon which this value was based, indicated that this BMDL0.5 could be in the range of 2.0 to 7.0 μg/kg bw/day (FAO/WHO, 2011).
18. It should be noted that the majority of the epidemiological studies from which the EFSA and JECFA BMDLs have been derived have focused on exposures to iAs via drinking water. To use the data generated by these epidemiological studies in their BMDL modelling, both Committees had to estimate dietary exposures for the study populations.
19. EFSA and JECFA established different BMDL values as they used different approaches in some aspects of their modelling; in particular, when modelling the dose-response data, and in their approaches to assessing dietary exposure to iAs of the populations in the epidemiological studies. JECFA also included studies in their modelling that had been published after the 2009 EFSA opinion.
20. The most recent assessment undertaken by EFSA in 2021 on dietary exposures of iAs found that after a review of the new data, mean dietary exposure among the adult population ranged from 0.03 to 0.15 μg/kg bw/day, and 95th percentile dietary exposure estimates ranged from 0.06 to 0.33 μg/kg bw/day. The major contributions to iAs exposure were rice-based products, rice, grains, and grain-based products along with drinking water. Overall, mean dietary exposure estimates were below the BMDL01 range values of 0.3–8 μg/kg bw/day established by the EFSA CONTAM Panel in 2009. In comparison to the previous assessment undertaken by EFSA in 2014 (EFSA, 2014) maximum mean and 95th percentile dietary exposure estimates for iAs were approximately 1.5-3 times lower across the age groups studied. The 2021 assessment also included ad hoc dietary exposures and found that breastfeeding provided reduced exposure to iAs compared to replacement rice-based formula. Exposures to iAs via exclusive breastfeeding were shown to be 0.04 µg/kg bw/day and 0.06 µg/kg bw/day for mean and high exposures respectively. Exposure via rice-based formula ingestion was estimated at 0.30 and 0.39 μg/kg bw/day in mean and high consumers, respectively. A review of exposure estimates to iAs for lactating and pregnant women showed mean values ranging from 0.03 and 0.06 µg/kg bw/day and 0.04 to 0.14 µg/kg bw/day respectively for each group. The 95th percentile exposures to iAs showed values between 0.08 and 0.25 µg/kg bw/day in lactating women and 0.09 and 0.28 µg/kg bw/day in pregnant women. Women in this category showed a similar pattern of food contributing to iAs intake as that of the general adult population (EFSA, 2021).
21. The COT has commented on As in food several times in the past for example in 2008 and 2016. In general, the conclusions, based on the available data, have been that dietary exposure to organic As was unlikely to constitute a risk to health, but that dietary exposure to iAs should be as low as reasonably practicable (ALARP) since it is genotoxic and a known human carcinogen (COT, 2008).
22. The most recent statement published by the COT in 2016 reported on the potential risks of As in the diet of infants and young children. The COT concluded that the JECFA BMDL0.5 of 3.0 μg/kg bw/day identified for lung cancer should be used in the characterisation of the potential risks from exposure to iAs. This was because the JECFA 2011 risk assessment was based on more robust and recent evidence than that available to EFSA in 2009. The focus of the risk characterisation was on iAs since this is the carcinogenic form. An MOE approach was used to compare the exposure estimates to the BMDL. The COT also agreed with the Food Standards Agency (FSA) advice to toddlers and young children (aged 1-4.5 years) not to be given rice drinks as a substitute for breast milk, infant formula, or cows’ milk and that this advice should remain in place. The conclusion of the assessment found that total exposure to iAs, from dietary and non-dietary sources, in infants and young children aged 4 to 12 months and 1 to 5 years generally generated MOEs of less than 10 and could therefore pose a risk to health. The assessment made it apparent that in these age groups, dietary sources generally contribute more significantly to exposure than non-dietary sources such as soil and dust. The Committee, therefore, reiterated that efforts to reduce the levels of iAs in food and water should continue (COT, 2016).
23. Along with food, drinking water is one of the most important sources of exposure to As. A provisional guideline value of 10 µg As/L of drinking water has been established by the WHO, given the practical difficulties in removing As from drinking water, particularly from small supplies, and the practical quantification limit for As (between 1-10 µg/L). WHO state that it is feasible to achieve As concentrations of 5 µg/L or lower using any of several possible treatment methods. However, this requires careful process optimization and control, and a more reasonable expectation is that 10 µg/L should be achievable by conventional treatment methods. In countries where the guideline is difficult to attain, WHO states that every effort should be made to keep concentrations ALARP (WHO, 2022).
24. Arsenic and associated compounds have been considered by the IARC in 1979, 1987, 2002, and 2018. The most recent evaluation concluded that As and iAs compounds are carcinogenic to humans (Group 1) due to the finding of sufficient evidence for carcinogenicity, particularly in the cases of kidney, liver, and prostate cancer. Furthermore, the iAs complexes dimethylarsinic acid (DMAA(V)) and monomethylarsonic acid (MMA(V)) are possibly carcinogenic to humans (Group 2B). Arsenobetaine and other organic As compounds not metabolized in humans are not classifiable as to their carcinogenicity to humans (Group 3). IARC reports that trivalent iAs has carcinogenic effects in utero including, but not limited to, the mechanisms of disruption of the oestrogen receptor, glucocorticoid receptor, and other steroids signalling in vivo and in vitro, altered expression of genes and acquired resistance to apoptosis allowing survival of cells with DNA damage (IARC, 2018).
Hazard Identification
25. This section focuses on summarising papers that have been published since the COT last reviewed As (as part of the infant diet) in 2016, but also includes summaries of papers that had been included in that statement.
Absorption, Distribution, Metabolism, and Elimination (ADME)
Inorganic Arsenic Species
26. Inorganic As that has been ingested is shown to absorb in the body differently depending on the solubility of the As compound, the food matrix, and the presence of other compounds in the gastrointestinal (GI) tract. Water-soluble As species are found to be more easily absorbed than fat-soluble As species (EFSA CONTAM, 2009). In humans, iAs is rapidly cleared from the blood and is widely distributed to almost all organs (FAO/WHO, 2011).
27. Bolan et al. (2021) reviewed the intestinal permeability of iAs, cadmium (Cd), lead (Pb), and mercury (Hg) as influenced by chelating agents and gut microbes using in vitro GI/Caco-2 cell intestinal epithelium model. The study showed that there was a significant decrease in the permeability of iAs (as arsenic oxide – As(III)) by 7.5% as measured by the apparent permeability coefficient value (Papp). The presence of chelating agents, ethylenediaminetetraacetic acid (EDTA), and diphenylmethylsilyl ether (DPMS) decreased the permeability of iAs from 60% to 47.3% and 38.9% respectively. The chelating agents were found to form complexes with metalloids, decreasing intestinal absorption and therefore making them less permeable. Chelating agents were also shown to increase cellular retention of the metalloids. For the addition of gut bacteria, the transport of As decreased from 60% to 37.6%. The decrease in permeability is thought to be associated with either indirect intestinal sequestration by gut bacteria or direct protection through adsorption of the metalloid onto the bacteria’s surface. Both mechanisms decrease the intestinal permeability of As and hence mitigate As toxicity (Bolan et al., 2021).
28. Inorganic As is metabolised primarily by stepwise reduction of As(V) to As(III), this is followed by oxidative addition of methyl groups, although alternative pathways have also been proposed that include methylated arsenical glutathione metabolites. Ingested iAs is excreted as As(V) and As(III), and as the pentavalent metabolites MMA(V) and DMA(V), with lesser amounts of the trivalent metabolites’ methylarsonous acid (MMAA(III)) and dimethylarsinous acid (DMAA(III)), and thioarsenical metabolites. Previously it has been assumed that methylation of iAs was a detoxification route (Gebel, 2002), however, some data suggests that the simple organic As species MMA(III) and DMA(III) appear to be more toxic than iAs (As(III) and As(V)), and have high affinity for thiols and cellular proteins indicative of their chemical reactivity (FAO/WHO, 2011). Ozturk et al. (2021) confirmed these findings, stating that other metabolites such as trivalent mono- and di-methylated forms of As can block enzyme function and are highly genotoxic (Ozturk et al., 2022). MMAIII is not usually detected in foods (MMAV is a trace species found in some seafood and terrestrial foods), while DMAIII is a very unstable reactive species that is difficult to measure and is not detected in foods (DMAV is a minor species in seafood and some terrestrial foods) (EFSA CONTAM, 2009).
29. There are many proposed pathways for iAs methylation, but the primary site of methylation is the liver due to its mass and the first-pass effect of ingested As (Vahter, 2002). The first methylation pathway for iAs was proposed by Challenger in 1955 (Challenger, 1955) and happens via oxidative methylation and reduction reactions that alternate stepwise. The proposed mechanism showed the coupling of a methyl group to an As(III) atom, yielding a methylated arsenical compound As(V). Thus, oxidative methylation of inorganic As(III) produces MMAA. Reduction of As(V) in MMAA produces monomethylarsonous acid (MMAA(III)). MMAA(III) is then oxidatively methylated to DMA(V). Cullen et al. (1979) along with other studies (Tam et al., 1979; Yamamura and Yamauchi, 1980; Yamauchi and Yamamura, 1983) confirmed this proposal and discovered that biomethylating microorganisms formed both the intermediate and end products. An alternative method for methylation was proposed by Dheeman et al., (2014) involving catalysis of the methylation reaction by the use of enzymes. Studies using As methyltransferases (ArsM) found that the substrate could be positioned closer to a bound S-adenosyl-L-methionine (AdoMet) (a methyl group donor) when As(III) was bound to three cysteinyl residues. This facilitates the transfer of a methyl group to a bound As atom yielding MMAA as the product (Packianathan et al., 2018). The study indicated that this conformation change could be the first stage of the catalytic cycle. As the As atom in the bound product of the first methylation reaction is not oxidized during the methyl group addition, it remains bound to cysteinyl residues. Methylarsenic can be further methylated to produce dimethylarsonic acid (DMMAA(III)) (Thomas, 2021). One further mechanism of iAs methylation has been demonstrated in the presence of glutathione (GSH) a co-factor, and a methyl donor S-adenosylmethionine (SAM), and As methyltransferase (AS3MT) in the liver. iAs is converted to MMA(III), DMA(III), and DMA(V) which are excreted in urine (Khairul et al., 2017).
30. Studies have shown that iAs metabolism and its subsequent toxicity are dependent on individual variation in the capacity to metabolise iAs (Luo et al., 2018; Paul, Majumdar and Giri, 2015; Hsu et al., 2015). Engstrom et al (2007) investigated polymorphisms in six genes and their effect on urinary metabolite patterns after exposure of 147 women to iAs via drinking water. The study found that polymorphisms in several genes (e.g. AS3MT) play a large role in the variation of iAs metabolism in the population (Engström et al., 2007).
31. At neutral pH, As(V) oxyanions mimic and have structural similarities to phosphate. This results in competitive inhibition of several phosphate-utilizing enzymes in intermediary metabolism and oxidative phosphorylation. When phosphate levels are starved, cells then become more sensitive to As(V) which can be taken up by phosphate transporters (Yang et al., 2012). As(V) can substitute for phosphate in the reaction with glucose and gluconate to generate glucose 6 arsenate and 6 arsenogluconate which act as analogues for the phosphate derivatives of this reaction. During glycolysis, negative feedback results in glucose 6 arsenate binding to glucose 6 phosphate dehydrogenase, impeding hexokinase activity. The As(V) compound restricts the formation of adenosine triphosphate (ATP) due to the instability of the anhydride product as it is readily hydrolysed. The formation of the product is more unstable due to the longer length of the As-O bond when compared to the P-O bond in the phosphate product. Along with this, in the mitochondria, As(V) attaches to adenosine diphosphate (ADP) to form an unstable compound in the presence of succinate. The instability causes uncoupling of this intermediate compound and hence further reduces the synthesis of ATP. As(III) covalently binds to lipoamide (a cofactor of dihydrolipoyl dehydrogenase) by vicinal dithiols and sulfhydryl groups. This binding forms bidentate adducts which in turn deactivates dihydrolipomide dehydrogenase of the pyruvate dehydrogenase complex. Inhibition of oxidative phosphorylation follows and prevents acetyl coenzyme A (acetyl-co-A) from converting to pyruvate, hindering Krebs’ cycle and subsequent ATP production (Giri and Dey, 2017).
32. Inorganic As distribution has been reviewed in several studies and has been shown to readily pass through the placenta, along with its metabolites, in mammals (Lindgren et al., 1984; Willhite and Ferm, 1984) including humans (Concha et al., 1998; Hall et al., 2007) with similar exposure levels in mother and foetus. During late gestation, the main metabolite that was found to reach the foetus was DMA(V) in both plasma and urine (Concha et al., 1998). This data was supported by studies in mice, where As found in the foetal tissue was abundantly DMA(V) (Devesa et al., 2006). Arsenic metabolism was also found to increase during pregnancy resulting in increased exposure of iAs and MA(V) to the foetus in early gestation (Concha et al., 1998; Hopenhayn et al., 2003). In contrast to the rapid transfer of iAs to the foetus, very little iAs was excreted in breast milk despite high iAs exposures from drinking water in a cohort of mothers in Bangladesh (Concha et al., 1998). As the small amounts of As passing to milk are almost entirely in the inorganic form it seems likely that efficient maternal methylation of As protects against excretion in breast milk (Fängström et al., 2008).
33. Inorganic As as described by EFSA (2009) has also been shown to distribute in the bloodstream between the plasma and erythrocytes (Liu et al., 2002; Villa-Bellosta and Sorribas, 2008; Schuhmacher–Wolz et al., 2009). For most iAs species, elevated levels occur in the liver, kidney, spleen, and lung following exposure, but accumulation is shown to occur in keratin-rich tissues such as the hair, nails, and skin several weeks after exposure. Total tissue As accumulation (measured as the sum of iAs, MMA, and DMA) was found to be largest in the kidney followed by lung, bladder, skin, blood, and lastly liver. Methylarsonate was the predominant metabolite in the kidney, whereas DMA(V) was shown to be the predominant metabolite in the lung (Kenyon et al., 2008). Inorganic arsenic and its metabolites are readily excreted in the urine and bile with a preferential route in humans of urinary excretion. The composition of urinary iAs metabolites varies from person to person and has been interpreted to reflect iAs methylation efficiency. However, the primary form of iAs excreted in human urine has shown to be DMA(III) (Vahter, 1999).
34. Gardner et al., (2012) (from abstract) report that pregnancy increases iAs metabolism independent of genotype. Their study evaluated the effects of the methyltransferase genotype, pregnancy, and their potential interactions on iAs metabolism. A longitudinal study of the iAs metabolite pattern in urine (at approximately gestational weeks 8, 14, and 30) of 303 women exposed to iAs through drinking water and food in rural Bangladesh was undertaken. ‘Data were available on genotypes for 16 polymorphisms, combined as haplotypes, in three methyltransferases: arsenic(+III)methyltransferase (AS3MT) and DNA-methyltransferases 1a and 3b (DNMT1a and DNMT3b)’. Logistic quantile regression was used to determine any significant changes in metabolite pattern by haplotype. The four haplotypes seen for AS3MT and the three haplotypes for DNMTa showed significant influence on the metabolite pattern found in the urine of pregnant women. The effects were found to continue for the entire course of pregnancy and the haplotypes studied were found to not interact with pregnancy-related changes in the iAs metabolism phenotype. DNMT3b haplotypes were found to not significantly influence the metabolites found in urine. A decrease of 5.7% in MMA(V), the most risk-associated mono-methylated metabolite, was shown to be a pregnancy-related change. Changes in levels on MMA(V) due to the haplotypes AS3MT and DNMT1a were found to be between 1.6 and 5.3%. The decrease seen in the concentrations of MMA(V) was related to an increase in As methylation efficiency which increased over pregnancy. The genotype had less of an effect than pregnancy-related changes on As metabolite pattern overall (Gardner et al., 2012).
35. Gao et al., (2019) examined the determinants of iAs metabolism in a birth cohort study of 1613 pregnant women in Bangladesh following exposure to iAs. Arsenic and its methylated metabolites were measured in maternal urine at 14-16 weeks and 21-37 weeks. The study found that As methylation increased during pregnancy. This was shown via a decrease in iAs from 8.5% at visit 1 to 6.6% at visit 2 and an increase in DMA from 85.7% at visit 1 and 87.9% at visit 2 (no change was observed with %MMA for all mothers). It was also found that other variables, including BMI, age and education, only affected iAs methylation in early gestation. From analyses by study visits, iAs methylation efficiency was positively associated with gestational age at visit 1 but not at visit 2. The author stated that the physiological mechanism of these findings had not yet been fully elucidated(Gao et al., 2019).
36. Iwai-Shimada et al., (2019) performed a birth cohort study to assess the profile of prenatal exposure to tAs and other toxic elements by review of maternal blood, cord blood, and placenta for pregnant women (N = 649). The median concentration of As in maternal blood was found to be 4.06 ng/mL and 3.68 ng/mL in cord blood. The study found that concentrations of tAs in cord blood were significantly lower than those in maternal blood and placenta. Concentrations in maternal blood were found to not represent foetal exposure for As. The study determined that the use of cord blood may be more suitable than maternal blood (especially, in cases with no correlation between element concentrations in the maternal blood and cord blood) in the assessment of exposure and consequent health effects. Especially because the placental transfer of certain elements (e.g. Hg, As, Cd, and Antimony (Sb)) varies largely among individuals (Iwai-Shimada et al., 2019).
Organic Arsenic Species
37. There are currently limited data on the ADME of organic As species in humans. However, some studies have determined the basic fate of organoarsenicals in animal metabolism.
38. One of the main organoarsenicals found in seafood, particularly crustaceans is AB, with one study finding that AB is not metabolised by humans and passes through the body rapidly, and is excreted unchanged (Le, Cullen and Reimer, 1994).
39. In a study performed by Buchet et al. (1981) (from abstract), volunteers ingested a single oral dose of organic As (500 µg As) either as MA(V), or DMA(V). The amount of As excreted in urine after four days was 78% and 75% of the ingested dose respectively. This suggested a GI absorption of greater than 75% for pentavalent organoarsenicals. However, more recent data, upon review of urinary excretion, suggest that there is considerable individual variability in the absorption of arsenosugars (Raml et al., 2009).
40. Taylor et al. (2017) reviewed human exposure to organic As from seafood and described that arsenolipids and arsenosugars are shown to break down to form the major metabolite, DMA, which is excreted in urine (Raml et al., 2009). An investigation by Xue et al. (2017) reports that although ArsM is required for iAs methylation, it is not required to synthesise arsenosugars from DMA. The study also determined that DMA is a precursor for arsenosugar biosynthesis (Xue et al., 2017).
41. More recent research has discovered the transformation of dietary methylated As species and arsenosugars (from oxo arsenosugars to thioxo analogues in the small intestine) by salivary microbiota (Calatayud et al., 2018). The influence of microbiota on bioaccessibility and biotransformation of iAs in the GI tract has also been recently discovered (Lu et al., 2013; Yin et al., 2016; Yin et al., 2017).
42. Xiong et al. (2022) evaluated the potential interaction of the gut microbiome and subsequent arsenolipid metabolism in the GI. Overall, the study determined that arsenolipids were retained in a donor-dependent way, in the mucosal and bacterial compartments of the simulated human gut, with much of the retained As being lipid-soluble. The study found that there was a higher retention of arsenic-containing lipids including fatty acids (AsFA) in comparison to arsenic-containing hydrocarbons (AsHC). AsHC retained in the mucus and bacteria were found to be largely intact but with some conversion to the thioxo analogue by the colonic microbiota. Whereas AsFA were seen to be bio-transformed to the thioxo analogue with conversion to arsenic-containing fatty esters and alcohols, along with As-containing sterols and 2 unknown compounds. The arsenolipids reviewed in this study were found to be rapidly bio-transformed into several lipid-soluble products with unknown potential toxicity. Therefore, their impact on human health could not be assessed (Xiong et al., 2022).
43. Chávez-Capilla (2022) completed a review of previous studies of arsenolipid transformations and asessed this against gaps in the research. The review showed that currently, there are nine structural groups of arsenolipids (Francesconi, Stick and Edmonds, 1990; Rumpler et al., 2008; Taleshi et al., 2008; García-Salgado et al., 2012; Amayo et al., 2013; Viczek, Jensen and Francesconi, 2016; Glabonjat et al., 2017; Řezanka et al., 2019) with AsFAs and AsHCs being of most interest, due to their similarity in cytotoxicity to iAs (Meyer et al., 2014; Meyer et al., 2015) and evident toxicity in several body parts including human liver, bladder, and brain cells (Meyer et al., 2014; Meyer et al., 2015; Witt, Ebert, et al., 2017; Witt, Meyer, et al., 2017).
44. AsFAs and AsHCs have been shown to produce metabolites that are less toxic As species in human liver and c.elegans model systems. The chemical structures of the products suggets that these two classes of arsenolipids enter the citric acid cycle (Meyer et al., 2015; González de Chávez Capilla, 2018; S. M. Müller et al., 2018; Bornhorst et al., 2020). The consequences of their entry into this cycle are not fully understood and raise questions regarding As’ influence on depleting cells of the energy required for essential metabolic steps (Meyer et al., 2014; S. M. Müller et al., 2018).
45. The review by Chávez-Capilla (2022) also highlighted the impact of organic arsenicals on maternal and foetal health. New evidence has demonstrated the permeability of AsHCs across the blood-brain barrier and its subsequent disruption of this barrier in mammals (Müller et al., 2017;S.M. Müller et al., 2018). This evidence supports associations found between AsHC exposure, resulting neurodegenerative disorders, and negative effects on mechanisms for learning and memory in infants (Niehoff et al., 2016; Müller et al., 2017; S.M. Müller et al., 2018; Witt, Ebert, et al., 2017; Zheng et al., 2021).
46. Kubota et al. (2005) describe the placental transfer of AB in mammals considering the consequence of organic As exposure in pregnancy. Total As concentrations in the liver, kidney, muscle, and blubber of a female Dall’s porpoise were found to be 0.76, 0.69, 0.35, and 0.55 mg/kg wet weight, respectively, with the concentrations in tissues of the six-month-old foetus being 0.28, 0.23, 0.26 and 0.07 mg/kg wet weight, respectively. A review of the As species found that AB was the major compound in the liver, kidney, and muscle for both mother and foetal porpoise (ranging from 76 to 91 % of the total As in the tissues). DMA(V), arsenocholine (AC), MA(V), and an unidentified As compound were detected as minor constituents in the tissue of both the mother and foetus (Kubota et al., 2005).
Toxicity
Reviews of Toxicity of Arsenic
Inorganic Arsenic
47. EFSA (2009) describes the main adverse effects associated with exposure via ingestion of iAs, which affects several critical regions of the body including the cardiovascular, GI, and reproductive systems. Arsenic compounds in the +3 oxidation state are described to be more toxic than those in the +5 state (EFSA CONTAM, 2009).
48. The US Agency for Toxic Substances and Disease Registry (ATSDR) state that exposure to iAs can cause adverse health outcomes and affect multiple physiological systems, including the following: cancer, respiratory irritation, nausea, skin effects, neurological effects, peripheral vascular effects, high blood pressure, circulatory problems, and high doses leading to encephalopathy. Chronic exposure has been linked with an excess incidence of miscarriages, stillbirths, preterm birth, and infants with low birth weights (US ATSDR. Syracuse Research Corporation, 2007).
49. A review by Ratnaike (2003) discovered that acute exposure to iAs results in the clinically common effects of nausea, vomiting, colicky abdominal pain, and diarrhoea. These symptoms have been found to resolve in 12 hours and do not require recovery or treatment. Other acute effects discovered are psychosis, skin lesions, seizures, and cardiomyopathy along with additional common effects such as respiratory failure, encephalopathy, and pulmonary oedema. Acute iAs poisoning has been found to have a lethal dose ranging between 100 mg and 300 mg with the United States Risk Assessment Information System database stating that 0.6 mg/kg/day is the acute lethal dose (Opresko, 1992). However, long-term iAs toxicity has been shown to lead to more threatening effects including multisystem disease and malignancy. Health outcomes resulting from iAs toxicity varies between individuals in the population and different geographical areas. The onset symptoms of chronic iAs poisoning are non-specific symptoms such as abdominal pain, diarrhoea, and sore throat that follow on to a large range of other clinical features affecting the skin, GI, cardiovascular, neurological, genitourinary, respiratory, endocrine, and haematological systems (Ratnaike, 2003).
50. Ratnaike et al. (2003) reviewed the mechanisms of iAs toxicity. The review found that iAs can inactivate up to 200 enzymes, with the main involvement in DNA replication/repair and cellular energy pathways. Arsenic was found to be substituted for phosphate in compounds like ATP and free As has been shown to exhibit toxicity by creating reactive oxygen species which can cause DNA damage and lipid peroxidation (Cobo and Castiñeira, 1997). Reactive oxygen intermediates can enter redox cycling and disrupt metabolic activation processes.
51. A review completed by Tolins et al. (2014) confirmed that early life exposure to iAs is correlated to deficits in memory and intelligence in subjects shown from data gathered by fifteen epidemiological studies. The effects described have been shown to occur at exposures lower than the current recommended safety guidelines and have been found to present neurocognitive consequences later in life. The timing of exposure and the sex of the individual was shown to alter developmental neurotoxicity. Four of the epidemiological experiments studied did not show behavioural outcomes after exposure.
52. A review of the acute effects of As exposure as stated by the World Health Organisation (WHO) (2018) showed vomiting, abdominal pain, and diarrhoea followed by muscle cramping, numbness, and tingling of extremities and in extreme cases, death in adults. Chronic effects (from approximately 5 years or more) of exposure show reports of skin lesions, pigmentation changes, and cancers of the bladder, lungs, and skin. Chronic ingestion has been shown to lead to developmental effects, diabetes, pulmonary disease, and cardiovascular disease. The WHO also reports associations between adverse pregnancy outcomes and infant mortality concerning As exposure. Arsenic exposure in utero and early childhood have shown increased mortality due to cancers, lung disease, heart attacks, kidney failure, and impairment of cognitive development, intelligence, and memory (WHO, 2023). There is some evidence for the neurobehavioral effects of iAs exposure during childhood, at exposure levels occurring in areas with elevated concentrations in drinking water.
53. A more recent review by Tchounwou et al. (2019) describes similar effects of iAs toxicity as those previously reported by other authors. The review described the involvement of iAs in tumorigenesis, highlighting several new potential modes of action such as alteration in p53 expression, gene amplification, DNA repair, and DNA methylation. However, these mechanisms are not well characterised. The review discusses mechanistic studies showing that iAs can act as a co-carcinogen and stimulate cancer progression (Tchounwou et al., 2015). Arsenic has also been shown to be mutagenic, and clastogenic, affecting changes in the structure and number of chromosomes and chromatids. Inorganic As has an effect on growth factors, cycling proteins, extracellular signal-regulated kinase (ERK) signalling, and mitogenic markers by upregulating their processes. Modulation of microRNAs has been seen following exposure to iAs, consequently affecting the expression of genes involved in tumour suppression and acting as oncogenes.
54. Both the central nervous system and peripheral nervous system are affected by iAs exposure. Several mechanisms appear to play key roles in neurotoxic effects including apoptosis, thiamine deficiency, decreased acetylcholinesterase activity, and oxidative stress. Peripheral neuropathy induced by chronic As exposure showed to be more pronounced in sensory fibres versus motor fibres. Axon degeneration of the peripheral nerves in unmyelinated and myelinated fibres, was shown during a biopsy. The symptoms seen with high As exposure were often found to be non-specific but did show a dose-response relationship in several organs in the neurological system. (Mochizuki, 2019).
55. JECFA also commented on the toxicity of As and stated that drinking water supplies containing 1 mg/L or more of As and concentrations of 0.1 mg/L can cause toxicity. From this, the Committee concluded that ingestion of 1.5 mg/day iAs could cause chronic toxicity and that 0.2 mg/L could increase skin cancer risk by 5% over a lifetime. It is indicated that the toxicity of As is dependent on the route of administration, species of organism, solubility, and chemical form. Inorganic As is shown to cause toxic effects to most regions of the body with the metabolites MMA(V) affecting the GI tract, kidneys, thyroid and reproductive system, and DMA(V) on the kidneys, thyroid, bladder, and development of the foetus. In humans, the main adverse effects as described by JECFA (2011) are: cancer, where risk is enhanced by poor nutritional status; skin lesions (particularly hyperkeratosis, hyperpigmentation, and hypopigmentation); peripheral and central neurotoxicity; several cardiovascular effects; and diabetes (JECFA, 2011).
56. There are several proposed mechanisms of carcinogenicity of iAs, including oxidative damage, epigenetic effects, and interference with DNA damage repair, but not following direct reaction with DNA (EFSA CONTAM, 2009). Inorganic As could therefore be considered an indirectly acting genotoxic carcinogen (COT, 2016).
Organic Arsenic
57. A few data are available regarding the toxicity of organic As compounds such as AB and AC in humans, however, exposure to such compounds is not generally considered to be of toxicological concern (EFSA CONTAM, 2009). Upon review in 2011, JECFA similarly concluded that there were insufficient data to assess the toxicity of organic As and concluded that inorganic and organic species should be considered separately. However, JECFA did state that among populations that consume large quantities of seafood (approximate intakes of 0.05 mg/kg bw/day), no reports of adverse effects had been seen and that further experimental evaluation would be needed to assess the toxicity of organic species in humans (JECFA, 2011).
58. A review completed by Luvonga et al., (2020) described the toxicity of organoarsenicals and their potential adverse effects. The review states that in general, the lower the oxidation state of the organic compound the higher the toxicity and hence the higher the rate of methylation to produce arsenic species with lower toxicity. Hence the following As species are listed in decreasing degrees of toxicity: MMA(III), DMA(III), As(III), As(V), trimethylarsine (TMA+), DMA(V), MMA(V), trimethylarsine oxide (TMAO), AC, and AB. The toxicity of iAs compounds is well understood and their mechanisms well established, however, acute toxicity has not been seen for organic compounds such as arsenolipids and arsenosugars, and their potential mechanisms and toxicity are yet to be fully elucidated. It is thought that the toxicity of these compounds arises from metabolism and the formation of more toxic products. The review also included consideration of toxicity by individual species. AC is known to be a precursor for AB synthesis and does not break down to iAs, MMA, or DMA, and hence, is considered to be benign. Similarly, AB is found to be a non-toxic As species and is a stable compound, resisting hydrolysis and metabolism in humans, being eliminated from the body intact. Arsenosugars are more susceptible to degradation/ metabolism in the body and are more chemically labile than AC and AB. Arsenosugars are found to be significantly less cytotoxic when compared to iAs, However, some arsenosugars, primarily in the trivalent oxidation state, have shown toxicity to the cell but these species have never been detected in biological systems. Arsenolipids have also been found to be a species of toxicological concern due to the similarities of their metabolites with iAs(III) which is a defined carcinogen (Luvonga et al., 2020).
59. Organoarsenicals and the implications of their toxicity were also reviewed by Xue et al. (2021). The review reiterates the proven toxicity of trivalent methyl arsenicals and describes their routes of toxicity including affecting cell viability by inhibiting enzyme activity, damaging the structure of the DNA, and activating proteins involved in cell proliferation, transformation and death. These As compounds in the trivalent state show greater genotoxicity and cytotoxicity than iAs and their pentavalent counterparts. The review also discusses the toxicity of arsenosugars and showed that many types only show minor toxicity in vitro even at high doses. However, using a Caco-2 intestinal barrier model, toxicity of arsenosugars and some metabolites (including thio-DMAs(V)) showed comparable cytotoxicity to iAs. Similarly, to arsenosugars, the review states that some arsenolipids have been shown to exhibit toxicity, mainly bioavailable AsHC compounds which can disrupt the neural network and cause apoptosis in the brain cells of humans.
60. The review by Chávez-Capilla (2022) detailed the previous studies that have evaluated organic As toxicity; however, data were reported to be scarce. Previous studies have stated that species such as AB and arsenosugars exhibit low toxicity (Ohta, Sakurai and Fujiwara, 2004; Leffers et al., 2013; Ebert et al., 2016). Arsenolipids have been shown to exert some toxicological effects. The arsenolipid subgroups, AsFA and AsHC, have shown similar toxicity to iAs due to their cytotoxic potential and toxicity seen in human liver, bladder, and brain cells (Meyer et al., 2014; Meyer et al., 2015; Witt, Ebert, et al., 2017; Witt, Meyer, et al., 2017). Regarding other organoarsenicals, limitations surrounding the method of isolation, synthesis, and analysis of these species have hindered further research in this area.
61. The remainder of this discussion paper will discuss the toxicity of As in the context of maternal health and pregnancy outcomes.
High Blood Pressure in Pregnancy
62. Andrews et al. (2022) sought to determine the associations between tAs exposure and changes in maternal blood pressure. Systolic and diastolic blood pressure were measured for 1,522 women every month from the time of enrolment onto the study up to delivery or miscarriage (up to 8 readings depending on gestational age). Participants from a hospital in Bangladesh were required to be of greater age than 18 years, have a confirmed singleton pregnancy of fewer than 16 weeks, and live at the same address for the length of the pregnancy. Throughout the study, participants provided toenail samples and drinking water samples at intervals. Toenail tAs concentrations were found to be between 0.03 – 40.6 mg/kg and drinking water tAs concentrations were between 0.5 – 1400 μg/L. Arsenic concentrations had an increasing dose-response relationship with maternal blood pressure for a gestational age of more than 25 weeks. Participants with a lower (<23 kg/m2) body mass index (BMI), at 40 weeks gestation, had a greater mean systolic and diastolic blood pressure by 2.83 mmHg and 1.96 mmHg, respectively when exposed to 50 μg/L tAs when compared to those exposed to ≤1 μg/L tAs. Participants with a higher (≥23 kg/m2) BMI had an increased mean systolic blood pressure of 5.72 mmHg and diastolic blood pressure change of 6.09 mmHg at 40 weeks gestation following exposure to 50 μg/L compared to those exposed ≤ 1 μg/L As. Women with a higher BMI showed larger differences in mean blood pressure at differing levels of As exposure when compared to women with a lower BMI.
63. Wang et al. (2021) investigated the relationship between iAs exposure and As metabolism with blood pressure changes and hypertensive disorders of pregnancy (HDP). A sample of urine was collected at intervals during each trimester from a total of 1,038 pregnant women (52 HDP, 986 non-HDP participants). Arsenic metabolism was evaluated using the resulting percentages of iAs, MMA, and DMA in urine and correlating the outcomes of HDP and systolic, diastolic, and mean arterial pressure changes during pregnancy. A higher DMA concentration in urine was associated with a lower increase in systolic blood pressure and arterial pressure compared to the reference participants that had lower concentrations of DMA in urine (< 7.19 µg/L). A positive association was found with the highest percentile of iAs in urine and weekly change of systolic blood pressure, diastolic blood pressure, and arterial pressure. The findings suggested that As metabolism and exposure may affect blood pressure changes among pregnant women.
Pregnancy Outcomes
64. Punshon et al. (2015) studied the relationship between placental tAs concentration and maternal and newborn exposures in a cohort of 766 pregnant women exposed to As via drinking water. Placental As levels were positively correlated with As levels in maternal postpartum toenail samples, gestational urine, and infant urine. Placental As concentrations were positively associated with drinking water As levels, with the model predicting a 1 μg/L increase in water As concentration increasing placental arsenic by 2.1%. Placental concentrations of As were suggested to reflect newborn and maternal exposures.
65. Stone et al., (2021) reviewed the current literature on adverse pregnancy outcomes and potential explanations for their effects. The review highlighted the effects of tAs exposure on the developing foetus with an explanation of a study by Fei et al., (2013) which demonstrated that tAs in urine caused increased expression of the AQP9 As transporter found in placental cells. Therefore, increasing the cytotoxicity of As in the placenta. The study by Fei et al. (2013) also found that an increase in AQP9 caused a decrease in ENPP2 expression. ENPP2 is an enzyme for the catalysis of a series of reactions that ultimately affect cell surface receptors involved in the regulation of early embryonic development, embryo implantation and spontaneous preterm birth, along with other effects. ENPP2 was also found to be associated with maternal hypertension. The review by Stone et al., (2021) also highlighted several studies that had demonstrated a link between iAs exposure and cases of preterm birth (Almberg et al., 2017; Huang et al., 2018; Shi et al., 2015).
66. Shih et al. (2017) sought to investigate the effect of maternal creatinine-adjusted urinary tAs concentration and related adverse pregnancy outcomes (stillbirth, spontaneous abortion, and therapeutic/ elective abortion) on an individual basis. This study reflected tAs exposure from multiple sources (water, food, soil, and dust), giving a more representative exposure than previous studies that have only evaluated exposure from a single source. Of the 489 births recorded in a cohort selected from a population in rural Bangladesh, 109 adverse pregnancy outcomes were recorded (18.3%) including 23 stillbirths (3.9%) and 60 spontaneous abortions (10.0%). Higher prenatal urinary tAs concentrations were associated with an increased risk of adverse pregnancy outcomes. Modelled as a continuous exposure measure, a 50 µg/g increase of As in urine resulted in a 2% increase in adverse outcomes overall and a 2% increase in stillbirth. Increased tAs was also related to an elevated risk of infant mortality. As a continuous exposure measure of As, a 50 µg/g increase in As concentration in urine resulted in a 4% increase in child mortality overall. Subset analysis evaluating the association with infant mortality showed 12 infant deaths before the age of 1 year were observed equating to a 7% increase using continuous exposure modelling at a level of 50 µg/g increased tAs in urine. Higher urinary tAs concentrations were seen to have higher risks of adverse pregnancy outcomes. These data agree with the previous meta-analysis (Quansah et al., 2015), studies, and reviews (Ahmad et al., 2001; Cherry, 2008; Hopenhayn-Rich et al., 2000; Milton et al., 2005; von Ehrenstein et al., 2006). However, some studies have not demonstrated an association between stillbirth (Myers et al., 2010; Rahman et al., 2010) or spontaneous miscarriage (Bloom et al., 2014) following elevated levels of tAs exposure above background concentrations.
67. Wang et al., (2018) investigated serum As concentration by analysis of maternal blood samples and adverse pregnancy outcomes in a cohort of 3,194 mothers in a Chinese population. The study did not state the species of As being measured but from the methodology provided, it was assumed that tAs was measured with no indication of an iAs concentration split. The women were categorised into two groups depending on the concentration of As in serum in accordance with the 75th percentile of serum As concentration: low-As group (L-As, ≤ 6.68 μg/L) and high-As group (H-As, > 6.68 μg/L). Low birth weight (< 2,500 g) showed an incidence of 2.2% in the L-As group and 2.9% in the H-As group (p = 0.25). No association with serum As concentration was determined for low birth weight incidence for either boys or girls following subset analysis. A review of small for gestational age (SGA) (birth weight less than the 10th percentile) outcomes for both groups showed that SGA for the L-As group was significantly (p = 0.044) lower than SGA for the H-As group being 7.6% and 9.9% respectively. No association was found between SGA and exposure for boys upon subset analysis but the incidence in girls was found to be significantly different with 10.2% in L-As and 14.2% in H-As (p = 0.037). A review of preterm delivery outcome and relation to serum As concentration showed that preterm delivery was significantly increased in the H-As group compared to the L-As group with an incidence of 7.0% and 4.8% respectively (p = 0.016). It was also reported that moderate-to-late preterm delivery (between 32 to < 37 weeks) had incidences for L-As and H-As of 4.2% and 6.1% respectively, with a significantly higher incidence in the H-As group (p = 0.035). The authors claim these findings indicate that greater maternal serum As concentrations are positively associated with several adverse pregnancy outcomes (Wang et al., 2018).
68. Smeester et al. (2017) considered the connection between the concentrations of toxic metals, including As, in amniotic fluid and fetal gene expression (the species of As were not stated in the study but from reading the introduction it has been assumed that the review is of iAs). The mean concentration of As in amniotic fluid was 16.3 µg/L and ranged between 3.4 to 41.3 µg/L in a cohort of 42 participants. Multivariable models found that greater As levels in amniotic fluid were associated with increased expression levels of three gene types: Olfactory Receptor, Family 4, Subfamily S, Member 2 (OR4S2), Phospholipase C, Beta 1 (Phosphoinositide-Specific) (PLCB1), and Progesterone Receptor (PGR). These gene types have been associated with adverse birth outcomes and reproductive effects. OR4S2 has previously been linked to an increased risk of preterm birth when increased copies of variants occur (Biggio et al., 2015). Arsenic has been associated with upregulation of PLCB1, which plays an important role in extracellular signalling and can cause intrauterine growth restriction (Sitras et al., 2009). All genes shown to have increased expression with As exposure are involved in the key pathways that may be related to spontaneous preterm birth, showed increased expression due to As exposure. None of the metals evaluated in the Smeester et al., (2017) paper showed any association with adverse outcomes relating to gestational age.
69. Attreed et al., (2017) performed a systematic review to question the association between in utero exposure to As (species not given) and immunotoxicity, through cell-mediated and humoral immunity. As exposure has been reported in several studies to affect the humoral immune response (Rahman et al., 2011; Farzan et al., 2013; Saha et al., 2013; Kile et al., 2014; Heaney et al., 2015; Ser et al., 2015). Exposure to As has been shown to increase total immunoglobin G (IgG) levels in both mothers and non-mothers. However, in pregnant women, As exposure has been shown to impair transplacental transport of IgG, reducing the number of antibodies received by the foetus. Exposure to As has also been shown to increase susceptibility to viruses. The exposure to As “may affect antibody response differently, depending on the pathogen-specific vaccine target’. Another study (Cardenas et al., 2015) indicated that an increased urinary As level, increases the odds of antibody loss which decreases antibody protection over time. This allowed reactivation of the virus with an increasing As dose. In vitro studies have evidenced cell-mediated immune responses to As exposure which affects critical properties of functional cellular immunity. For example, As reduces interleukin-2 (IL-2) a cytokine that aids in the mediation of cellular immune function. A reduction in this cytokine is linked to decreases in T cell activation and proliferation. Arsenic is shown to alter the structure of T cells which in turn can decrease protein secretion. Epidemiological studies have marked evidence that suggests IL-2 decreases with increasing As exposure and that pro-inflammatory cytokines increase, resulting in increased cord blood T cell proliferation and alterations in the subset of cord blood T cells (Attreed, Navas-Acien and Heaney, 2017).
70. Zaw and Taneepanichskul (2019) explored how heavy metal exposure affects brain-derived neurotrophic factor (BDNF), a key molecule involved in the development of learning and memory and an important pregnancy biomarker (Miranda et al., 2019). The study determined that high As concentrations (concentrations above the median value of 0.44 µg/dL) found in maternal blood correlated with a 2.6-fold increase odds ratio of low BDNF in plasma in the first trimester of pregnancy when compared with a low blood tAs group. No association was found between low plasma BDNF, and high concentrations of the other metals (Pb, Hg, and Cd) tested in the experiment. Lower levels of BDNF have been associated with a significant impact on newborn neurodevelopment and maternal depressive disorders.
71. Richter et al. (2022) described associations between prenatal As exposure and the risk of congenital heart disease (CHD). The study reviewed iAs exposure via drinking water in a nationwide cohort study in Denmark with 1,042,413 births. The study did not explicitly state the species of As tested, however, the introduction and discussion review the effects of iAs, and hence it has been assumed that iAs concentrations were reviewed in this study. Prenatal As exposure was defined as the concentration of As in the drinking water at the address of the mother when the gestational age of the foetus was 4 weeks. The testing point of four weeks was selected as this is within the time critical period of 4 – 7 weeks when foetal cardiac development occurs. Median As exposure from drinking water for the cohort was 0.53 µg/L with a range between 0.0015 µg/L and 36.0 µg/L. CHD was found to be more prevalent in infants where prenatal exposure in drinking water was ≥ 5 µg/L compared to exposure < 5 µg/L, with 12.3 and 9.2 cases of CHD per 1,000 births seen respectively. The results showed a monotonic exposure-response relationship. For severe CHD, infants with high maternal exposure (≥ 5.0 μg/L) had a 0.5 case increase per 1,000 births compared to exposures at lower levels. A similar pattern was observed for septal defects, where increasing As exposure resulted in a 1.5 per 1,000 births increase in septal defects between the highest and lowest exposures. Valvular defects were shown to increase when exposure increased, resulting in a 0.4 increase in cases per 1,000 births. However, the finding for valvular defects was found not to be significant. The study found that overall, even at low concentrations (0.5-0.9 µg/L), maternal exposure to iAs increases the risk of CHD in infants.
72. Suhl et al. (2022) determined that prenatal tAs dietary exposure was strongly linked to several adverse non-cardiac birth defects. A case-control study was conducted with 10,446 control infants and 14,408 case infants in the United States where dietary exposure to tAs was divided into low, middle, and high tertiles among mothers (low <0·07, middle 0·07–0·21 and high ≥0·22 (μg/kg bw/day)). Middle and high tertile exposures showed a three-fold increase in cloacal exstrophy and a positive association with increased incidence of colonic atresia/stenosis, oesophageal atresia, bilateral renal agenesis or hypoplasia, hypospadias, cloaca; exstrophy, gastroschisis, and intercalary limb deficiency. High tertile As exposure was also linked to an increase in cases of encephalocele, glaucoma/anterior chamber defects, choanal atresia, intestinal atresia stenosis, and bladder exstrophy. Middle tertiles of iAs exposure were also linked with encephalocele, intercalary limb deficiency, and transverse limb deficiency. Other associations with birth defects were found to be inversely or null associated.
73. Navasumrit et al., (2019) studied tAs exposure in utero and its association with types of DNA and micronuclei damage in newborns. A cohort of 205 women in Vietnam was recruited for the study. Toenail and urine samples were collected at around 25 weeks of pregnancy and cord blood was collected post-delivery to assess tAs exposure. Various types of DNA damage were evaluated including presence of 8-hydroxy-2’-deoxyguanosine (8-OHdG), 8-nitroguanine, and DNA strand breaks. All types of DNA damage were shown to significantly increase with increasing maternal As exposure. Damage was measured by reviewing variation frequency in cord blood and this was found to increase in a dose-dependent manner. Micronucleus frequency in mononucleated cells also increased with increasing As exposure. The study authors concluded that the increase in 8-OHdG, 8-nitroguanine, DNA strand breaks, and micronucleus (MN) frequency in infants suggests that As transfers across the placenta and is susceptible to maternal exposure to As and its metabolites. This genetic damage in newborns can contribute to diseases, including cancer, during development and later life (Navasumrit et al., 2019).
74. de Assis Araujo et al. (2022). investigated prenatal exposure to tAs and other metals and consequent impairment to neurodevelopment in infants at six months. A study population of 48 newborns was selected from a hospital in Rio de Janeiro where tAs concentration was determined by analysis of maternal and umbilical cord blood and neurodevelopment was assessed using the Denver Development Screening Test II (DDST-II). The geometric mean for tAs concentration in maternal blood samples was found to be significantly larger (p= 0.03) in the ‘fail’ category of the DDST-II in comparison to the ‘not fail’ category. A review of subgroup failures discovered that personal social, fine adaptive motor, language, and gross motor domains were associated with higher maternal and cord blood tAs concentrations (although these were not significant). Overall, higher tAs concentrations in maternal blood were positively associated with failure of the DDST-II test (p=0.07).
75. The findings from the study by de Assis Araujo et al., (2022) were confirmed by an investigation conducted by Devick et al. (2022). This used Bayesian Kernel Machine Regression (BKMR) to ‘quantify the contribution of birth length as a mediator between in utero co-exposure to tAs, manganese, and lead, and children’s neurodevelopmental scores, in a prospective birth cohort in Bangladesh’. Exposure to the metals, including tAs, was measured by analysis of umbilical cord blood. A negative association was reported between metal exposure and neurodevelopment, with birth length mediating the effect of exposure to the metals. It was observed that when birth length was fixed at the 75th percentile, the effect of exposure was weakened suggesting that maternal nutrition and its impact on foetal growth and birth length, could mediate harmful exposure to metals and its impact on neurodevelopment (Devick et al., 2022).
76. An analysis by Wang et al., (2018) determined the effects of tAs exposure and consequences on neonatal neurobehavioral development for a cross-sectional study of 892 pregnant women and their infants in Shanghai, China. Neurodevelopment was predicted using the neonatal behavioural neurological assessment (NBNA) with newborns at 3 days of age. Newborns with higher cord serum tAs concentrations (median concentration = 3.93 µg/L) were shown to have a lower NBNA score than those who scored higher on the NBNA test (median concentration = 0.61 µg/L). It was found that cord blood As levels were significantly inversely associated with passive muscle tone, behaviour, and total NBNA score. A one natural log unit increase in cord blood arsenic levels were associated with 90% increased odds of a low NBNA score. The study also discovered that newborns born to older mothers (age >29 years) were more susceptible to having lower neurobehavioral performance after exposure to tAs. However, other cohort studies conducted in Bangladesh did not find an association between maternal As exposure and motor and behavioural outcomes in children of varying ages up to 18 months (Tofail et al., 2009; Hamadani et al., 2010).
77. A study completed by Ahmed et al., (2019) assessed the connections between chronic maternal tAs exposure via drinking water and neonatal mortality/foetal loss. A prospective cohort study performed in Bangladesh analysed 1,574 mother-infant pairs where tAs exposure was measured using maternal urine samples. The study found that overall, there was not a significant association (p= 0.208) between As exposure and offspring death. However, a time-varying association with mortality was discovered. For those with increased As exposure, mortality decreased in the early stages of pregnancy and increased after 24 weeks gestation (although not statistically significant) which was then followed by a decrease in mortality that approached null at the late stages of pregnancy. The time-varying association was also observed when the data was modelled as a step function following adjustment for covariates (such as maternal age, monthly income, and maternal education) where mortality decreased with increased tAs exposure until an approximate gestational age of 20 weeks, after which, mortality increased as tAs increased. The study authors concluded that non-linear association found by this study suggested that As toxicity may vary depending on the gestational age of the foetus and that exposure to As during early gestation could invoke survival pressure of the developing foetus and hence contribute to survival bias (Ahmed et al., 2019).
78. Winterbottom et al. (2019) described how increasing tAs exposure affects the biological functions of the foetal placenta and consequently foetal health and development. The study analysed 46 infants from a cohort study undertaken in New Hampshire. Prenatal As exposure was assessed by collection of maternal urine where urinary tAs was measured from samples collected at 24 - 28 weeks. The analysis of urine also measured individual As species due to the high levels of tAs found in the samples and therefore tested for As(III), As(V), DMA(V), DMAA(V), MMAA, and AB. However, AB was excluded from the analysis due to its perceived nontoxicity. RNA sequencing was used to analyse changes in gene expression by review of placental samples. Upon review of results, the study found that differential expression did not affect any female genes using a false discovery rate (controlling the expected proportion of falsely rejecting the null hypothesis) of < 0.05 although associations were found with LEMD1 and UPK3B with 2.51- and 2.48-fold changes in expression following higher tAs exposure. However, 606 genes were expressed differentially in males compared to females, with FIBIN and RANBP3L having the greatest association with higher tAs exposure with 0.14- and 0.15-fold changes respectively. Gene set enrichment analysis showed that 211 gene sets in the female placenta and 154 in the male placenta were enriched with differentially expressed genes following higher tAs exposure. In the female placenta, 103 of the gene sets were linked with lower weights at birth. The research found that overall, tAs could affect multiple biological mechanisms in the placenta and that a subset of gene expression effects is sex dependent.
79. Deyessenroth et al., (2022) used maternal toenail clippings to measure tAs concentration and its effects on alterations of placental gene transcript proportions and associated birth weight differences. Placental samples (n=199) were reviewed to determine placental transcriptome and single nucleotide polymorphisms. Small for gestational age infants had 82 genes that were associated with differential transcript usage (DTU) where the gene ORMDL1 showed DTU association with increased exposure to As. The study authors concluded that these changes suggest that increased in utero exposure to tAs and genetic variants play a role in impacting fetal growth through disturbances in placental mechanisms (Deyssenroth et al., 2022).
80. Research undertaken by Wei et al., (2018) ‘aimed to explore the role of metabolites in mediating the association of arsenic exposure on infant birth weight’. Study samples from a cohort in Bangladesh comprised of 20 mothers and 35 matched newborn pairs. Inorganic As exposure was reviewed using maternal toenail samples taken in the first trimester and foetal exposure was assessed via cord blood samples. Metabolomic profiles were created by evaluation of 20 maternal peripheral blood samples and 35 cord blood samples. The level of iAs in cord blood was positively associated with an elevation in the levels of 17-methylstearate, laurate, and 4-vinylphenol sulphate. An increase in iAs was also shown to correlate with lower birth weights. In the second trimester, two peripheral blood metabolites (butyrylqlycine and tartrate) were associated with lower cord serum iAs. Intrauterine and maternal peripheral blood metabolites were found to influence the toxicity of iAs in relation to lower birth weight by preventing metabolic disruption, including fatty acid pathways that impact birth weight.
81. Kile et al. (2015) explored the relationship between birth weight and gestational age and tAs exposure in a cohort study based in Bangladesh using structural equation models. Total As exposure was assessed using measurements from drinking water samples and maternal toenail clippings and was found to have a mean concentration of 2.3 µg/L in water and a median concentration of 1.46 mg/kg in toenail samples. Drinking water tAs concentration was strongly correlated with toenail As concentration. The results of the study showed that a ln-unit increase in water As concentration was linked to a decrease in birth weight. The decrease in birth weight was mediated by reduced maternal weight gain during pregnancy and earlier delivery. Models reviewing maternal As toenail concentration drew similar conclusions. Govarts et al. (2016) found similar results for tAs exposure in single pollutant models and its effect on infant birth weight as did several other studies where increasing concentrations of tAs in cord blood resulted in decreased birth weight, even at low levels of exposure (Guan et al., 2012; Xu et al., 2011).
82. Abdel Hameed (2020) performed a cross-sectional study of 113 mother-newborn pairs to assess iAs exposure (the species was not explicitly stated by the authors, but the introduction implies that it is a review of iAs) during pregnancy, using maternal and cord blood samples, to evaluate their associations with adverse birth outcomes. The study revealed that maternal serum As concentration was significantly negatively associated (p= 0.04) with gestational age and similarly associated with the newborn Apgar score at 1 minute and 5 minutes (Abdel-Hameed, 2020). The Apgar score measures the overall condition of a newborn 1 minute and 5 minutes post birth including checks for activity, pulse, grimace, appearance, and respiration.
83. The study by Abdel Hameed (2020) showed no association between iAs concentration and head circumference. Conversely, Davis et al. (2015) found that increasing maternal urinary tAs concentration was associated with a decrease in foetal head circumference, with a stronger association found with the female foetus at approximately 18 weeks of pregnancy. A 1 µg/L increase in tAs concentration resulted in effect estimates for head circumference decreasing by 0.047 and decreasing biparietal head diameter Z-score by 0.072 (no units) for female foetuses.
Effects on Maternal Health
84. In a study conducted by Chen et al. (2011), the association between tAs exposure from drinking water and the occurrence of proteinuria was assessed. Proteinuria is a well-recognised marker for increased risk of chronic renal disease. A positive association was found between urinary tAs and proteinuria which showed a dose-response relationship. The proteinuria prevalence odds ratio increased above 1 for urinary As levels above 36 µg/l and rose to 1.82 for women with the highest tertile of As exposure.
85. Susko et al., (2017) completed a retrospective study to determine the relationship between low-level iAs exposure and female fecundity. The study was conducted with 94 women from a cohort in Romania who were asked to report the number of menstrual cycles they experienced while trying to conceive, this waiting time until recognised pregnancy was also referred to as the time to pregnancy (TTP) and was used as a measure of biological capacity to reproduce. The study found a moderately lower probability of conception in women that experienced longer TTPs compared to women with shorter TTPs, and further compared to unexposed (average drinking water As level of 0 µg/L) women. When consuming an average of 1 µg/L of iAs in drinking water, the 6th, 9th, and 12th menstrual cycles showed a 5%, 8%, and 10% lower probability of pregnancy respectively. The study authors concluded that iAs exposure may impair female fecundity but highlighted the need for confirmation of this hypothesis by a more definitive study (Susko et al., 2017).
86. Liang et al., (2020) measured the concentrations of tAs in maternal and cord serum and respective maternal and neonatal thyroid hormone parameters in a cohort of 2,089 mother-newborn pairs in China. The concentration of As was determined in all three trimesters of pregnancy along with the thyroid hormone parameters: thyroid stimulating hormone (TSH), and free thyroxine (FT4). Multiple linear regression was applied to explore the associations between As exposure and thyroid hormone parameters. The study found that in all trimesters, exposure to As was not associated with maternal thyroid hormone parameters following adjustments for covariates. However, an inverse association was found between neonatal FT4 levels and average As exposure, with the study authors concluding that this potentially demonstrates that the effect of As on the foetus is not mediated by the mother (Liang et al., 2020).
87. A review by Ishfaq Ahmad et al., (2021) discussed the literature surrounding the reproductive toxicity of As. Data has shown that iAs disrupts the neuroendocrine system due to the inhibition of oestrogen binding receptors and the dysregulation of progesterone receptors. This disruption can cause diseases such as endometriosis that affect fertility. Inorganic As exposure has also been shown to cause problems concerning angiogenesis in the endometrium during pregnancy, causing problems during embryo development. Both of these changes can lead to premature birth, spontaneous abortions, endometrial dysfunction, sterility, and subfertility (Milton et al., 2017). Arsenic (species not stated by author) can affect the brain, pituitary, and germ line cells that are linked with the reproductive system, causing degenerate ovarian follicles, and decreased levels of progesterone and oestradiol. The low levels of oestradiol cause prolonged dioestrus and a decline in the number of oestrus cycles (Ghersevich et al., 1994). Inorganic As can interrupt hormone synthesis, for example, iAs prevents the conversion of androgen to oestrogen in oestrogen biosynthesis (Davey et al., 2008). Arsenic (species not stated in paper but references alude to review of iAs) also inhibits the enzymes involved in progesterone synthesis which can lead to poor conception and interferes with dopamine beta-hydroxylase activity which hinders a cascade of events that ultimately impedes ovulation (Kim et al., 2014). Ovulation disruptions can manifest in irregular or absent menstrual periods and cycle abnormalities. Another method by which arsenic impacts fertility is by directly affecting the uterus. Methylated arsenicals have been shown to decrease the weight of the uterus which in turn can increase pre-implantation embryonic loss (Ma et al., 2003).
88. The effects of tAs exposure and preeclampsia have been published by several authors. Liu et al. (2022) (from abstract) determined that the mean tAs concentration in blood was positively associated with the incidence and severity of preeclampsia, where an increase of the effect was seen in an increasing concentration-dependent manner. The study also reviewed blood markers that predict the occurrence of preeclampsia and evidenced that with increasing As concentration, an increase in hypoproteinaemia and mean corpuscular haemoglobin concentration occurs, with a decrease in mean corpuscular volume of red blood cells being primary indicators. Sandoval-Carrillo et al. (2016) studied a cohort of 104 women in Mexico with varying levels of tAs exposure via drinking water, ranging between 2.48 and 76.02 µg/L which is over the maximum limits set by WHO (10 µg/L) and those set in Mexico (25 µg/L). Due to the differences in concentrations, the participants were split into three groups depending on the concentration of exposure. Although tAs exposure over 25 µg/L presented an increased risk of preeclampsia, the difference was not statistically significant (p = 0.214). These findings were similar to that of other studies, one by Bommarito et al., (2019) who found that the association between urinary As and preeclampsia had a hazard ratio of 0.73 and Maduray et al., (2017) who found no statistical significance (p=0.5) between As concentration and preeclampsia. However, a study by Wang et al. (2020) contradicted these previous findings using a cohort of 427 women with preeclampsia and 427 matched controls. In single-pollutant models, tAs blood concentration in the ‘middle’ (9.97–20.62 µg/L) exposed group found preeclampsia to be more prevalent (with an odds ratio of 1.64) when compared to matched controls without preeclampsia from Maiyan, China.
89. Zargari et al., (2022) reviewed the literature surrounding As, its relation to oxidative stress, and subsequent effects on the reproductive system and infertility. An imbalance of reactive oxygen and nitrogen species (ROS/RNS) was found to cause oxidative stress, affecting several integral structures and functions of the cell and consequently adverse disorders such as diabetes, cancer, and infertility. However, low levels of ROS are fundamental for fertility as they are produced and required during sperm-oocyte interaction. Inorganic As has been shown to alter biogenic amines that control oogenesis and modify reproductive hormones (Jana, Jana and Samanta, 2006). Inorganic As has also been shown to affect enzymes involved in steroidogenesis, an important pathway in ovarian cells that maintain reproductive tissues, establish pregnancy, and regulate ovarian function. Inorganic As (sodium arsenite) has been found to reduce plasma gonadotropin which in turn reduces the activity of 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase which are essential for the regulation of steroidogenesis (Zargari et al., 2022).
Animal Studies (Reproductive Toxicity)
90. Kabir et al. (2020) investigated the mechanism by which iAs exposure impedes embryonic development. Zebrafish (Danio rerio) embryos were exposed to sodium arsenate at concentrations of 1.0 mM, 1.5 mM, and 2.0 mM, and embryogenesis was then reviewed under a microscope. The impacts of iAs exposure to breeding performance are measured by review of spawning, fecundity, fertilization rate, and hatching rate with the success rates for each being 80%, 82%, 75%, and 95% respectively in a baseline sample group of 20. With increasing As exposure at 1, 1.5, and 2mM, hatching period was increased (from 48-72 hours to 96-120 hours) and hatching percentage was significantly decreased (p<0.001), from 100% to approximately 20, 15, and 5% respectively. Exposure to ≥ 1.0 mM of As showed evidence of developmental and morphological abnormalities such as deformations to hatching gland cell, otic (auditory) vesicle, egg mass, tail bud, and midbrain with the most notable abnormality being pericardial oedema and dorsal curvature. Heart rate was also noted to dose-dependently reduce with increasing iAs exposure. The greatest effects of exposure were seen between the concentrations of 1.5 mM and 2.0 mM for all parameters studied.
91. Chen et al., (2022) exposed 40 female Sprague Dawley rats to iAs via drinking water using sodium arsenite concentrations of 0, 0.02, 0.2, or 2 mg/L from 3 weeks of age until 65 days of age (approximate exposure for 44 days) to assess the effects on the reproductive system. Exposure was shown to disrupt the oestrous cycle with more disturbance as iAs levels increased. Reproductive organ development was measured and showed no difference in the overall body weight of the rats compared to the controls, however, both ovarian weight and uterus weight dose-dependently decreased in groups of higher concentrations evidencing that As affected reproductive development without hindering overall animal growth. There was also an effect found on follicle reserves (atretic follicle increase and primordial follicle decrease) in the ovaries and reduced thickness in the endometrium of the uterus. Reproductive hormones were monitored and on exposure to all concentrations of iAs, there was a significant decrease in ovarian steroid hormones (estradiol (E2), progesterone (P4), and testosterone(T)) and increased pituitary/hypothalamus hormones (follicle-stimulating hormone (FHS), luteinizing hormone (LH), and gonadotrophin-releasing hormone (GnRH)) in dose-dependent manners. In addition, iAs selectively down-regulated the signalling molecules, phosphorylated protein kinase A (PKA), phosphorylated extracellular signalling-regulated kinase (ERK), c-Jun N-terminal kinases (JNK), Jun proto-oncogene AP-1 transcription factor subunit (cJUN), suggesting that ‘arsenic-dependent reductions in multiple steroidogenic proteins may be mediated, at least partially, through PKA-ERK/JNK-cJUN signaling pathway. Several ovarian steroidogenic-related proteins were affected including follicle stimulating hormone receptor (FSHR), steroidogenic acute regulatory protein (STAR), Cytochrome P450 family 17 subfamily A member 1 (CYP17A1), 3β-hydroxysteroid dehydrogenase (HSD3B1), and CYP19A, where reduction in CYP19A could result in fewer or lower expression of ovarian theca cells, since CYP17A1 is specifically expressed by ovarian theca cells the reductions could result from either fewer theca cells developed or lower expression level by each cell. Inorganic As showed an influence on reproductive capacity by means of smaller pups, smaller litter sizes, and a reduction in the number of male pups with no change to female pups (Chen et al., 2022).
92. Monaco et al., (2018) exposed 12 pregnant rats (at each concentration) during pregnancy and lactation to 0.05 and 0.10 mg/L iAs from drinking water ad libitum and measured the resulting cognitive deficits effects on short and long-term memory in the adult female offspring using inhibitory avoidance testing. The rats from each group were treated from gestational day zero, determined by the presence of spermatozoa from a vaginal smear to post-natal day 21. The group exposed to an iAs concentration of 0.10 mg/l showed a significant reduction of long-term memory retention but did not show an effect on retrieval for short-term memory when exposed to any iAs concentrations during pregnancy and lactation. To investigate the mechanism in which long-term memory is affected, α7 nicotinic acetylcholine receptor (a7-nAChR) mRNA expression in the hippocampus was measured against exposure. Significant differences were seen following exposure where females had a decrease in a7-nAChR mRNA levels. Oxidative stress on the brain and hippocampus was investigated as another potential cause of decreased long-term memory by observed levels of anti-oxidative enzyme activity (catalase (CAT) and glutathione peroxidase (GPx)) and lipid peroxidation (malondialdehyde (MDA) content). A decrease in CAT activity levels was observed in mothers, with no significant effect on GPx. A significant decrease in MDA was observed in pups following iAs exposure at both 0.05 and 0.10 mg/L. (Mónaco et al., 2018).
93. Neurotoxic effects were also observed by Chandravanshi et al., (2019) (from abstract) who exposed rats to 2 or 4 mg/kg body weight to iAs (species of As not stated in the abstract but references of the paper allude to review of iAs) to assess subsequent dysfunction of cholinergic and dopaminergic receptors. The study found that perinatal exposure to all As concentrations tested in the study induced hypo-activity due to impairment of protein expression and ultimately reduced spatial learning and memory, to below the average, in a dose-dependent manner. Learning and memory were also found to be impeded due to reduced expression of the cholinergic receptor muscarinic 2 (CHRM2) receptor gene and protein expression of choline acetyltransferase (ChAT), protein kinase C beta type 1 (PKCβ-1) in the frontal cortex and hippocampus (Chandravanshi, Gupta and Shukla, 2019).
94. A review by Nath Barbhuiya, Barhoi and Giri (2021) was undertaken to assess the impact of iAs on the reproductive health of female rats and mice. One study described how iAs at 50, 100, and 200 mg/L in drinking water for 28 days affects reproductive organs by decreasing the diameter of the uterus and the height of the epithelium with a reduction in the thickness of the endometrium and myometrium (Akram et al., 2010). This was observed in other studies following exposure to 0.4 ppm (0.4 mg/L) sodium arsenite from drinking water for 28 days coupled with other effects such as undefined endometrial lumen which was evidenced to be narrow and unfolded indicating reproductive damage (Elshawarby et al., 2014). Arsenic (administered orally at 10 mg/kg body weight for 8 days) reduced CAT, GPx, and superoxide dismutase (SOD) activity in rats along with induction of DNA breakage and necrosis of the uterine tissue and disruption of steroidogenesis (Dash et al., 2018). Another study by Davila-Esqueda et al., (2012) reported decreased serum oestradiol levels and degeneration of DNA in the ovaries following exposure of between 0.4 - 0.5 mg iAs/kg/day per rat per day from drinking water (Dávila-Esqueda et al., 2012). Inorganic As (given at 8 mg/kg bw/day once every other day for 16 days) was also seen to increase ROS in the ovaries of rats (Wang et al., 2017) and disrupt the oestrous cycle by increasing the length of the dioestrous and metestrus phases with an increase in follicular atresia following exposures at 10, 30 and 50 µg/L for 60 days (Mehta and Hundal, 2016). Rats showed uterine disturbance at oral exposures of 4 µg/ml for 28 days by means of changes in the levels of gonadotropins and oestradiol levels, sequentially causing the luminal epithelial, myometrial, and stromal cells to disintegrate. This was accompanied by downregulation of components of the oestrogen signalling pathway (Chatterjee and Chatterji, 2010). Arsenic exposure (at 0.4 – 0.5 mg iAs/kg/day via drinking water from conception to pup age of between 2 and 4 months old) demonstrated effects on puberty causing delayed onset, decreased follicular genesis and changes in the morphological characteristics of the ovaries and modified adrenocortical cell number (Dávila-Esqueda et al., 2012). Lastly, exposure of mice to iAs after administration of 0, 0.2, 2, and 20 ppm in drinking water (assumed mg/L) administered to parents from 35 days before breeding and continued through pregnancy until weaning induced autophagy in the ovaries by activation of autophagic genes including pyruvate dehydrogenase kinase 1 (PDK1), tuberose sclerosis 2 (TSC2), phosphatidylinositol 3-kinase (P13K), autophagy related 13 (ATG13), AMP-activated protein kinase (AMPK), Unc-51 like autophagy activating kinase 1 (ULK1), autophagy related 12 (ATG12), autophagy related 5 (ATG5), microtubule-associated protein light chain 3 (LC3), Beclin1, autophagy related 3 (ATG3), autophagy related 7 (ATG7), and sequestosome-1 (SQSTM1) and proteins (Beclin1, microtubule-associated protein 1A/1B-light chain 3 (LC3-I, II), and the mammalian target of rapamycin (mTOR)), decreasing the number of female gametes when compared to the control group (Ommati et al., 2020).
Biomarkers of Arsenic Exposure
95. In epidemiological studies, arsenic exposure has been estimated using various biomarkers. Many studies have determined the significant positive correlation between total and speciated forms of As exposure and subsequent levels found in urine and nail samples but the use of blood, hair, urothelial, and oral mucosa cells have also been reported. Some biomarkers have also been able to assess genotoxic and biochemical effects but are not widely used due to the specificity and the cost of testing (Marchiset-Ferlay, Savanovitch and Sauvant-Rochat, 2012).
96. Fei et al. (2013) investigated in utero tAs exposure to determine if certain placental gene expression is related to tAs levels. A cohort of 133 pregnant women was screened for gene expression for candidate genes that are involved in As metabolism, transport, and disease susceptibility along with As-regulated target genes such as aldo-keto reductase family 1 member C3 (AKR1C3), ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2), heme oxygenase 1 (HMOX1), leptin (LEP), nuclear factor erythroid-derived 2-like 2 (NFE2L2), thymidylate synthase (TYMS), aquaporin 9 (AQP9), arsenite methyltransferase (AS3MT), and solute carrier family 39 (zinc transporter) member 2 (SLC39A2). A positive association was found between expression in the placenta of the As transporter AQP9 and maternal urinary tAs concentration. AQP9 was seen as a potential foetal biomarker for tAs exposure.
97. Newborn mitochondrial DNA copy number (mtDNAcn) was measured following in-utero tAs exposure by Song et al. (2020). Seven hundred and sixty-two mother-infant pairs were selected from a cohort in China where maternal urinary tAs levels in each trimester was measured and compared to cord blood mtDNAcn. The median urinary tAs concentrations were 7.2 mg/L, 16.0 mg/L, and 17.0 mg/L in the first, second, and third trimesters respectively. No association was found in the second, and third trimesters but using multivariate modelling, a 6.6% decrease in mtDNAcn in cord blood was associated with each doubling of urinary tAs level in the first trimester.
98. Clark et al., (2022) measured the biomarkers of As methylation efficiency and birthweight and how these are modified by maternal serum concentrations of one-carbon metabolism factors: folate, vitamin B12, and homocysteine. Maternal exposure to tAs of 200 pregnant women was determined by urinary concentrations of iAs and its metabolites, MMA and DMA. Foetal exposure was measured similarly with iAs, MMA, and DMA in cord blood. Inefficient arsenic methylation was indicated by a higher MMA concentration in maternal urine. The percentage of urinary MMA was seen to decrease with increased levels of folate and vitamin B12 in maternal serum and with lower levels of homocysteine. The study authors concluded that differences in the level of one-carbon methylation factors caused differential rates of MMA to DMA conversion and are associated with As methylation efficiency. The authors suggested that “this represents a potential mechanism through which maternal diet may modify the harms of prenatal exposure to iAs” (Clark et al., 2022).
Hazard Characterisation
99. The dose response modelling and derivation of a health-based guidance values (HBGVs) by EFSA and JECFA, were reviewed by the COT in its 2016 statement for As in the infant diet. It was decided by the COT that the JECFA BMDL0.5 of 3.0 μg/kg bw/day identified for lung cancer should be used in the characterisation of the potential risks from exposure to iAs (COT, 2016). The study used to determine this value is summarised in the following paragraphs.
Benchmark Dose Modelling
100. An association between As and lung cancer was observed in a cohort study in Taiwan and was the basis for the BMDL0.5 determined by JECFA in 2011 and accepted by the COT in 2016. This study was chosen by JECFA over a previously conducted case-control study for lung cancer, as the earlier study showed possible selection bias in the hospital-based control group. Other studies were also considered including incidence of urinary tract cancer and skin lesions; however, these were not selected due to substantial differences in the studies concerning the case definition, method of exposure assessment, and assessment of potential confounders (eg. sun exposure and smoking). Due to these differences, these studies were not selected for dose-response modelling (JECFA, 2011).
101. The study conducted by Chen et al., (2010) evaluated cumulative exposure to iAs via drinking water from shallow wells (< 40 m in depth) over 11 years for 8,086 participants aged ≤ 40 years from 4,586 households of 18 villages. All participants were interviewed via questionnaire and information such as cigarette smoking, demographic characteristics, habitual alcohol consumption status, and residential and well water consumption history were recorded. Total dietary exposure (i.e., from food and water) was not evaluated in this study.
102. Arsenic exposure from well water was analysed using hydride generation and flame atomic absorption spectrometry. The concentrations determined by this analysis ranged between undetectable (< 0.15 µg/L) and 3,000 µg/L. The cumulative iAs exposure for the participants was calculated using the sum of As exposure (µg/L) and the number of years of drinking well water from the start to the end of consumption (defined as either the participant had stopped drinking well water or the end of the study if still drinking well water) from this source. The incidence of lung cancer was determined by a review of national cancer registry profiles and newly diagnosed cases within the study period.
103. The statistical significance of the duration of well water consumption, the median level of As concentration, and cumulative iAs exposure by selected demographic characteristics were examined using Kruskal-Wallis or two-sample Wilcoxon rank-sum tests. Arsenic exposure was divided into five categories depending on the iAs concentration in well water (< 10, 10 – 49.9, 50 – 99.9, 100 – 299.9, and ≥ 300 µg/L). The categories were used to emphasise the significance of the risk of lung cancer at lower iAs concentrations. The study also stated that there would be some measurement error in the cumulative iAs exposure group of zero due to trace amounts of iAs present in all water ingested by humans.
104. The relative risk and confidence intervals (95%) used estimation from Cox’s proportional hazard regression models. The final model included categorical adjustments for gender, age, cigarette smoking status, schooling year, and alcohol consumption. ‘Trends across levels of categorical variables were assessed by testing the statistical significance of a single trend variable coded as the category of exposure’. Sensitivity analysis was also performed as several study participants were farmers and had previous exposure to iAs via the use of pesticides on crops. Therefore, the sensitivity analysis was applied to include only those who reported that they had no previous exposure to iAs via pesticide use.
105. The results showed that those participants who were still drinking well water containing 10 µg/L or more of iAs at the time of enrolment or drank well water from birth had an increased risk of lung cancer of approximately 30% (RR = 1.32, 95% CI: 0.87, 1.98 for drinking from birth; RR = 1.28, 95% CI: 0.90, 1.83 for still drinking). Statistically significant dose-response trends (still drinking (p=0.002), drinking well from birth (p=0.027), not drinking from birth (p=0.009)) were seen when three different reference groups were used (reference groups of 0, < 100, and < 400 µg/L water concentration). The study also reports an approximately 2-fold increase in risk for the highest cumulative exposure of > 10,000 µg/L.
106. The study found that there was a significant dose response identified between the risk of lung cancer and increasing iAs concentration. The risk of lung cancer was not seen to increase over the 11-year study for those drinking water containing iAs between 10 µg/L and 100 µg/L. However, at iAs concentrations of 100 µg/L to 300 µg/L evidence of excess risk was displayed (RR 1.54, 0.97-2.46) and those drinking iAs concentrations above 300 µg/L showed a relative risk of 2.25 (95% CI: 1.43, 3.5) when compared to the reference group of <10 µg/L. Small cell carcinoma (p = 0.021) and squamous cell carcinoma (p = 0.004) showed significant dose-response relationships between increasing iAs concentration and increased prevalence of lung cancer.
107. The dose-response relationship was less prevalent in those participants that stopped drinking well water (p = 0.115) when compared to those who still drank well water at enrolment (p = 0.002). Lung cancer risk was similarly associated with those who started ingesting well water from birth and those who started post-birth (ages greater than age 0-20). Cigarette smoking and increased iAs exposure also showed a large increase in the risk of lung cancer. Participants who had consumed water containing ≥ 100 µg/L iAs and smoked ≥ 25 packs of cigarettes per year (over the study period) showed to have a 7-fold increased risk than those participants that drank water containing iAs <10 µg/L and had never smoked cigarettes (RR = 6.97, 95% CI: 3.4, 14.3). Analysis of the combination of iAs concentration and the durations of exposure determined that participants that had been drinking high iAs concentrations (≥ 300 µg/L) for over 50 years at the time of the study resulted in a 10-fold increased risk of lung cancer (RR=9.71, 95% CI: 2.84, 33.2) than those who drank low concentrations (<10 µg/L) over 30 years or less.
108. To formulate the BMDL0.5, the incidence of lung cancer was used by JECFA (FAO/WHO, 2011), and the data generated was fitted with nine dichotomous models using modelling software (BMDS v 2.1.1). From this, the lowest BMDL0.5 of 3.0 µg/kg bw/day was produced by modelling from quantal-linear regression. The approach for a quantitative assessment of risk of iAs was determined only using concentrations of iAs from drinking water and hence, conversion from these values to total dietary exposure was required. To produce this value, assumptions were made regarding exposure to iAs ‘from food before cooking and the volumes of drinking-water consumed directly and in cooking for the populations in which the respective 34 health endpoints were studied’. Average exposure estimates were used for volumes of water and food consumed to extrapolate to iAs exposure from food and water due to the uncertainties regarding actual exposure. From this, a range of low to high exposure values were calculated and considered the dietary habits of the iAs concentration in food of the region being reviewed. Sensitivity analysis showed that the BMDL0.5 could be in the range of 2.0 – 7.0 µg/kg bw/day with an assumption that iAs exposure through drinking water (including water used in cooking) has a greater impact than exposure through food (FAO/WHO, 2011).
Exposure Assessment
Exposure from Food
109. A large proportion of occurrence data collected for food safety assessment report tAs without differentiation of organic and inorganic species. Therefore, as tAs is considered as being exclusively iAs, potentially considerable overestimations have been made in assessments when considering dietary As exposure and its associated health risks. This has raised the need to acquire data related to species speciation (EFSA CONTAM, 2009).
110. The Food Standards Agency Exposure Assessment Team provided dietary exposure data for inorganic and total As for women of childbearing age (16 – 49 years), as shown in Appendix 3. Total mean dietary exposure for iAs and tAs was calculated by the exposure assessment team using the mean exposure for the food groups listed in Appendix 3 and the consumption of women of childbearing age (as a proxy for pregnant women) as taken from the Total Diet Study (TDS) (FERA, 2015).
111. The food groups with the highest contribution of iAs in the diet are miscellaneous cereals (which includes rice and rice products) and potatoes with a mean exposure of 0.027 and 0.016 µg/kg bw/day respectively and 97.5th percentile exposure of 0.076 and 0.048 µg/kg bw/day respectively. The highest estimated mean contributor for tAs in the maternal diet is from the food groups fish and seafood, miscellaneous cereals, and poultry with a mean exposure of 0.74, 0.018 and 0.0062 µg/kg bw/day respectively, and 97.5th percentile exposure of 3.6, 0.053 and 0.022 µg/kg bw/day respectively.
112. The total mean dietary exposure of iAs was estimated as 0.043 - 0.26 µg/kg bw/day (Lower bound (LB) – Upper bound (UB)) and 97.5th percentile exposure as 0.098 - 0.51 µg/kg bw/day (LB – UB). The total mean exposure of tAs was estimated as 0.77 - 0.89 µg/kg bw/day (LB – UB) and 97.5th percentile exposure of 3.6 - 3.8 µg/kg bw/day (LB – UB).
113. The data presented in Appendix 3 on occasion shows values for tAs that are lower than the values for iAs for the same commodities. This is due to a large number of non-detect values for As in the metals survey and therefore in the exposure assessment. However, this is consistent with As results and analysis as seen in previous COT reviews.
Exposure from Drinking Water
114. Data for tAs in drinking water was obtained from the Drinking Water Inspectorate (DWI) (for England and Wales) and the Drinking Water Quality Regulator (DWQR) for Scotland and Northern Ireland Water. The concentration data from 2018 to date for tAs in drinking water is given in Table 2.
115. The analysis of exposure to As from drinking water assumes that all tAs present in the samples are iAs. This is to make a conservative assumption in terms of toxicity to compare to the JECFA BMDL0.5 of 3.0 µg/kg bw/day that measured the incidence of lung cancer following iAs exposure.
Table 2: Mean and standard deviation (SD) concentrations of tAs (assumed all iAs) in tap water sampled in nations of the UK from 2018 to date (µg/L).
|
Region |
N |
LB Mean As Concentration |
LB SD As Concentration |
UB Mean As Concentration |
UB SD As Concentration |
|
England and Wales* |
49638
|
1.96 |
2.04 |
2.04 |
1.97 |
|
Scotland |
1398 |
0.06 |
0.18 |
0.35 |
0.14 |
|
Northern Ireland |
1568 |
0.10 |
0.18 |
0.32 |
0.06 |
LB = Lower Bound: values below the limit of detection assumed to be zero.
UB = Upper Bound: values below the limit of detection assumed to be the same as the limit of detection.
*99th percentile concentration.
116. The FSA Exposure Assessment Team has provided values for water consumption for women of childbearing age. These are a median of 8 g (ml) water per kg bw/day and a 97.5th percentile of 32 g (mL) water per kg bw/day. Using the upper bound concentration values from Table 2, exposure to tAs in drinking water is shown in Table 3.
Table 3: Median and 97.5th percentile exposure values for women of childbearing age to tAs (assumed all iAs) from drinking water, using the mean upper bound occurrence concentration values (µg/kg bw/day) from nations of the UK.
|
Region |
N |
Median As Exposure** |
97.5th Percentile As Exposure** |
|
England and Wales* |
49638 |
0.00023 |
0.00093 |
|
Scotland |
1398 |
0.000040 |
0.00016 |
|
Northern Ireland |
1568 |
0.000040 |
0.00015 |
*Occurrence values from 99th percentile concentrations.
**Average body weight of 70.3 kg for women of childbearing age used for exposure calculation. Value provided by the FSA EAT Team from years 1-11 of the rolling NDNS.
Exposure from Air
117. The Department for Environment, Food and Rural Affairs (Defra) provided data on air pollution throughout the UK using an interactive map. This shows that the majority of the UK had an average air concentration of 0.6 ng As/m3 or below with a large proportion of the central areas of the UK having average concentrations of 0.61 and 1.2 ng As/m3. Smaller pockets in the north of England show average concentrations between 1.3 and 2.4 ng As/m3 (Department for Environment Food Rural Affairs, 2020).
118. The WHO estimates that the average inhalation rate for a 70 kg adult is 20 m3/day (WHO, 2000). As a worst-case scenario, if an adult female were to be continuously exposed to 2.4 ng As/m3, this would result in a daily exposure of 48 ng As from the air. For women with an average body weight of 70.3 kg (value provided by the FSA Exposure Assessment Team from years 1-11 of the rolling National Diet and Nutrition Survey) (Public Health England, 2014, 2016b, 2018, 2020) this gives an exposure of 0.68 ng/kg bw (0.00068 µg/kg bw/day).
119. These values assume that there is full absorption of all As in particles inhaled. However, this is dependent upon particle size and whether some of the inhaled doses becomes trapped in other parts of the nasopharynx. The inhalation values calculated are conservative but may contribute in a small proportion to the way As is ingested.
Exposure from Soil and Dust
120. Exposure to As can be increased by swallowing soil and dust that contains As. Ingestion of contaminated soil is often a result of “hand-to-mouth” activity and is a more important route of exposure for toddlers and children. However, this still presents a potential source of intake in adults, for example, through the surface of unwashed vegetables.
121. Arsenic concentrations in soil are influenced by underlying lithological As concentrations and anthropogenic release. Arsenic was measured in topsoil from England and Wales from a depth of 0-15 cm as part of a Defra-commissioned project (Ander, Johnson and Palumbo-Roe, 2011; Ander, MR Cave and Johnson, 2013).
122. The analysis of exposure to tAs from soil assumes that all As present in the samples are iAs. This is to make a conservative assumption in terms of toxicity to compare to the JECFA BMDL0.5 of 3.0 µg/kg bw/day that measured the incidence of lung cancer following iAs exposure.
123. Table 4 displays the tAs concentrations and exposures from soil for women of childbearing age. Mean and 75th percentile As concentrations from the soil in regions of England are defined as principal, ironstone, and mineralised and in Wales as principal, urban, and mineralised to assess potential exposures of adults through soil ingestion. An ingestion rate of 50 mg soil/day was assumed based on the rate used by the Environment Agency in their Contaminated Land Exposure Assessment (CLEA) model (Jeffries and Martin, 2009) and was based on a consensus value from studies by the United States Environmental Protection Agency (USEPA) (US EPA, 1997) and Otte et al., (2001). It is a combined value for soil and dust as most of the evidence used to determine the ingestion rate does not differentiate between soil and household dust. Furthermore, the evidence bases for selecting a representative soil ingestion rate for adults is much smaller than that for children, and as such USEPA (1997) cautioned that the value is highly uncertain and based on a low level of confidence.
Table 4: 75th percentile exposure values for women of childbearing age to tAs from soil. Soil tAs concentrations taken from the Defra-commissioned contaminants in the soils of England report (Ander, Johnson and Palumbo-Roe, 2011; Ander, MR Cave and Johnson, 2013) and ingestion of 50 mg soil/day provided by the Environment Agency (2009).
|
Region |
Area Name |
Mean Soil Concentration (mg/kg) |
Mean percentile Arsenic exposure (µg/kg bw/day)* |
75th Percentile Soil Concentration (mg/kg) |
75th percentile Arsenic exposure (µg/kg bw/day)* |
|
England |
Principal |
16 |
0.011 |
19 |
0.014 |
|
England |
Ironstone |
73 |
0.052 |
83 |
0.059 |
|
England |
Mineralised |
181** |
0.130 |
106 |
0.075 |
|
Wales |
Principal |
24 |
0.017 |
24 |
0.017 |
|
Wales |
Urban |
83 |
0.059 |
93 |
0.066 |
|
Wales |
Mineralised |
33 |
0.023 |
33 |
0.023 |
Urban: The three South Wales Valleys.
Principal: All samples not assigned to another domain.
Ironstone: Areas where underlying ironstones supply high levels of As. Must have >15% iron oxides.
*Calculated (to 2 significant figures) using mean and 75th percentile data and 50 mg soil/day ingestion. Average body weight for women of childbearing age used for ingestion rate is 70.3 kg. This value is provided by the FSA Exposure Assessment Team from years 1-22 of the rolling National Diet and Nutrition Survey (NDNS) (Public Health England, 2014, 2016b, 2018, 2020).
**NOTE: Although mean concentration value is higher than 75th percentile, this is correct against the Ander report.
124. The data presented in Table 4 are representative of tAs concentrations in soil in England and Wales only. There are currently no available Defra data for tAs levels in the soil of Scotland and Northern Ireland.
125. Pica behaviour is described as the craving for intentional ingestion of substances that are not described as food. Globally, it is thought that pica affects up to 28% of pregnant women, although with a high degree of geographical variability (Fawcett, Fawcett and Mazmanian, 2016). Pica presents a potential route of exposure to As in the maternal diet. However, pica has not been considered as part of this statement due to the lack of data available for the consumption of soil as part of pica behaviour.
126. No recent data were available for the levels of As measured in household dust in the UK.
Aggregate Exposure
127. Aggregate exposure to As from food, drinking water, soil, and dust, and air was derived by consideration of a number of scenarios based on available data.
128. Aggregate exposure to tAs and iAs from the diet, drinking water, air, and soil for women of childbearing age is presented in Table 5. The estimates were derived by combining 97.5th percentile exposure from the TDS (FERA, 2015) with mean exposure from all other sources (drinking water, air, and soil). Dust was not considered as there were insufficient available data.
Table 5. Aggregate exposure (µg/kg bw/day) to tAs and iAs for women of childbearing age based on data from the total diet (P97.5), drinking water, air, and soil (it is assumed that all As in drinking water, soil, and air are iAs).
|
Species of As |
Total Diet (LB to UB) a |
Drinking water * |
Air |
Soil ^ |
Total (LB to UB) |
|
iAs |
0.098 – 0.51 |
0.00023 |
0.00068 |
0.017 |
0.12 – 0.53 |
|
tAs |
3.6 – 3.8 |
0.00023 |
0.00068 |
0.017 |
3.6 – 3.8 |
Values have been rounded to two significant figures.
a The values are from the TDS (FERA, 2015) reported in Appendix 3, Tables 12 and 13, which also includes a negligible contribution from tap water.
*Exposure value is based on the highest average for drinking water (from England and Wales data).
^ Exposure value is based on the highest mean for a principal location.
129. The data in Table 5 shows that relative to the diet, drinking water, air, and soil make a negligible contribution to total exposure.
Risk Characterisation
130. Potential risks from maternal exposures to As were characterised using a MOE approach, calculated as the ratio of the BMDL0.5 of 3.0 µg.kg bw/day identified for lung cancer (FAO/WHO, 2011) to estimated exposures from diet, soil, and air.
131. While there is a widely accepted precedent for the interpretation of MOEs that have been calculated based on a BMDL for a 10% increase in the incidence of tumours in experimental animals, there is no such precedent for the interpretation of MOEs based on epidemiological studies of human cancer, in which reliable estimates of cancer incidences appreciably less than 10% are often available for use as the benchmark response. The Committee on Carcinogenicity of Chemicals in Food, Consumer Products and the Environment (COC) has advised that such MOEs should be considered on a case-by-case basis (COC, 2022).
132. In interpreting the MOEs calculated for iAs, the COT (2016) noted that the BMDL that had been used was for a 0.5% increased risk of lung cancer in humans, and was based on data from a recent, well-conducted, prospective cohort study (Chen et al., 2010). Chen et al., (2010) reported that the cancer risk increased with the duration of exposure, which was typically in the order of decades in their study. Furthermore, the Committee noted that iAs does not appear to exhibit direct genotoxicity; it appears instead to exhibit genotoxicity as a secondary effect, potentially following primary effects such as oxidative damage, epigenetic effects, and interference with DNA damage repair. For these reasons, the Committee agreed that in this case a MOE of 10 or above would be considered of low concern (COT, 2016).
Food
133. Taking the BMDL0.5 of 3.0 μg/kg/bw/day identified for lung cancer reviewed by JECFA and accepted by COT in 2016, the MOEs for women of childbearing age from the highest As-containing dietary items are given in Tables 6 and 7. Exposure values for these commodities can be found in Table 12 in Appendix 3.
Table 6: Calculated MOEs against a BMDL0.5 of 3.0 μg/kg/bw/day for iAs exposure in the food groups with the highest measured mean arsenic exposure (upper bound) for the total diet in women aged 16 to 49 years of age.
|
Commodity |
Mean As exposure (µg/kg bw/day)* |
MOE for 3.0 µg/kg bw/daya |
|
Miscellaneous cereals |
0.027 |
110 |
|
Potatoes |
0.016 |
190 |
*Average body weight for women of childbearing age used for exposure= 70.3 kg, value provided by the FSA Exposure Assessment Team from years 1 –11 of the rolling National Diet and Nutrition Survey, NDNS (Public Health England, 2014, 2016b, 2018, 2020).
aValues given to 2 significant figures.
Table 7: Calculated MOEs against a BMDL0.5 of 3.0 μg/kg/bw/day for iAs exposure in the food groups with the highest measured 97.5th percentile arsenic exposure (upper bound) for the total diet in women aged 16 to 49 years of age.
|
Commodity |
97.5th As exposure (µg/kg bw/day)* |
MOE for 3.0 µg/kg bw/daya |
|
Miscellaneous cereals |
0.076 |
40 |
|
Potatoes |
0.048 |
63 |
*Average body weight for women of childbearing age used for exposure= 70.3 kg, value provided by the FSA Exposure Assessment Team from years 1 –11 of the rolling National Diet and Nutrition Survey, NDNS (Public Health England, 2014, 2016b, 2018, 2020).
aValues given to 2 significant figures.
134. The total mean dietary exposure of iAs was estimated as 0.043-0.26 µg/kg bw/day (LB-UB) and 97.5th percentile exposure as 0.098-0.51 µg/kg bw/day (LB-UB). Using the upper bound values for exposure for the mean and 97.5th percentile, the MOEs calculated are 11.5 and 5.9 respectively.
135. The mean and 97.5th percentile MOEs for iAs for foods with the highest measured levels of iAs as reported by the NDNS have a value greater than 10, indicating that the iAs in these foods would indicate only a low safety concern for women of childbearing age. However, for total mean dietary exposure, a MOE of less than 10 (5.9) is observed for the 97.5th percentile data and therefore, there could be a potential concern for health for this group.
136. Although ‘fish and seafood’ show high mean and 97.5th percentile exposure results, 0.74 and 3.6 µg/kg bw/day respectively (Table 13 in Appendix 3), the high levels of tAs found in fish and seafood are likely to be from the contribution of organic As species. Most As in fish (> 90%) is in the form of AB which is also the main form found in crustaceans and bi-valve molluscs (Kohlmeyer, Kuballa and Jantzen, 2002), the remainder is AC and a small amount of iAs (usually < 1%). Therefore, as AB is the most abundant proportion of As in tAs, and is regarded as lower toxicity, there would be less of a concern for human health.
Drinking Water
137. Taking the BMDL0.5 of 3.0 μg/kg established by JECFA and accepted by COT in 2016, the MOEs for tAs in drinking water are shown in Table 8. Using this approach, it is assumed that As in water is entirely iAs to draw conclusions using a worst-case scenario.
Table 8: Calculated MOEs against a BMDL0.5 of 3.0 μg/kg/bw/day for As in drinking water using the concentration data provided by the water regulators for England and Wales, Scotland, and Northern Ireland and consumption data provided by the FSA Exposure Assessment Team.
|
Region |
97.5th percentile As exposure (µg/kg bw/day) ** |
MOE for 3.0 µg/kg bw/daya |
|
England and Wales* |
0.00093 |
3,200 |
|
Scotland |
0.00016 |
19,000 |
|
Northern Ireland |
0.00015 |
20,000 |
*99th percentile concentration occurrence data used.
**Calculated using 97.5th percentile data. Average body weight for women of childbearing age used for ingestion rate is 70.3 kg. This value is provided by the FSA.
Exposure Assessment Team from years 1-22 of the rolling National Diet and Nutrition Survey (NDNS) (Public Health England, 2014, 2016b, 2018, 2020).
aValues given to 2 significant figures.
138. The MOEs for intake of As in drinking water from four countries of the United Kingdom are all considerably greater than 10, and therefore, acute, and chronic toxicity is unlikely from this route of exposure.
Air
139. The inhaled exposure level would have minimal impact upon As exposure. Assuming all of the reported concentrations in air are iAs as a worst-case scenario, relative to the BMDL0.5, a conservative intake from the air gives a MOE of 4,400 (to 2 significant figures).
Soil and Dust
140. The MOEs for the exposure from As in soil are shown in Table 9. Using this approach, it is assumed that As in soil is entirely iAs to draw conclusions using a worst-case scenario.
Table 9: MOEs against a BMDL0.5 of 3.0 μg/kg/bw/day for As in soil from regions in England and Wales using the mean concentrations of As. Soil As concentrations taken from the Defra-commissioned contaminants in the soils of England report (Ander, Johnson and Palumbo-Roe, 2011; Ander, MR Cave and Johnson, 2013) and ingestion of 50 mg soil/day provided by the Environment Agency (2009).
|
Region |
Area Name |
Arsenic ingestion (µg/kg bw/day)* |
MOE for 3.0 µg/kg bw/daya |
|
England |
Principal |
0.013 |
230- |
|
England |
Ironstone |
0.059 |
51 |
|
England |
Mineralised |
0.075 |
40 |
|
Wales |
Principal |
0.017 |
180 |
|
Wales |
Urban |
0.066 |
45 |
|
Wales |
Mineralised |
0.024 |
130 |
* Average body weight for women of childbearing age used for ingestion rate = 70.3 kg, value provided by the FSA Exposure Assessment Team from years 1 –11 of the rolling National Diet and Nutrition Survey, NDNS (Public Health England, 2014, 2016b, 2018, 2020).
aValues given to 2 significant figures.
141. The MOEs calculated for soil ingestion across England and Wales are greater than 10 therefore, any risks of adverse health effects from As in soil are likely to be small. Furthermore, the soil ingestion rate is likely to be conservative, particularly in combination with dust. The ingestion rate is highly uncertain as it is based upon a small and variable evidence base. Consequently, the actual soil ingestion rate and As exposure through this route could be much lower.
Aggregate Exposure
142. A combined exposure assessment, considering exposure to iAs from all sources, relative to the JECFA BMDL0.5 of 3.0 µg/kg bw/day, gives an MOE of 5.7 calculated using the 97.5th percentile exposure upper bound values of iAs as presented in Table 5. There could be some concern of adverse health effects from the aggregate exposures. However, this is likely to be driven by iAs exposure from the diet, which was considered using 97.5th percentile exposure. It has already been identified that there could be some potential concern for adverse health effects form this contributing source when the entire diet is considered and for high consumers (97.5th percentile). Contributions from drinking water, air and soil and dust were mean exposures and were identified as low concern.
Conclusions
143. Arsenic is a heavy metal pollutant that is abundant in the environment and is present in the general diet of the population, including women of childbearing age through ingestion of foods such as fish, seafood, and rice. Arsenic is proven to exist in different forms and many species have been identified. These species can be found in food and are divided into organic and inorganic subgroups. The main food group identified to contain high levels of iAs were rice and rice products and fish and seafood for organic As, where the abundant form of the organic species present in fish is AB.
144. For the general population, As presents symptoms of exposure such as cancer, nausea/vomiting, respiratory problems, and skin lesions. Arsenic exposure in pregnant women has been shown to cause increased blood pressure, adverse pregnancy outcomes, and preeclampsia. For the developing foetus, As exposure has been linked with a higher incidence of stillbirth, spontaneous abortion, low birth weight, pre-term birth, altered gene expression, and birth defects along with a number of other adverse outcomes as outlined in this paper.
145. From a study by Chen et al., (2010), which measured iAs exposure via drinking from well water, JECFA (FAO/WHO, 2011) derived a BMDL0.5 of 3.0 µg/kg bw/day for a 0.5% increase in the incidence of lung cancer. The study reviewed the incidence of small cell carcinoma, squamous cell carcinoma, and adenocarcinoma from 8,086 participants over an 11-year period. The derivation of the BMDL0.5 was discussed at COT in 2016 and the committee concluded that the value should be used in the characterisation of the potential risks from exposure to iAs. (COT, 2016).
146. The COT previously concluded that a MOE of 10 or above would be considered of low concern when considering exposures to iAs (COT, 2016).
147. The review of tAs exposure showed that ‘fish and seafood’ had relatively high mean and 97.5th percentile exposure results. These levels are thought to be from the percentage of organic As in these species and the contribution from AB. Most arsenic in fish (> 90%) is in the form of AB which is also the main form found in crustaceans and bi-valve molluscs (Kohlmeyer, Kuballa and Jantzen, 2002), the remainder is AC and a small amount of iAs (usually < 1%). Therefore, as AB is the most abundant proportion of As in tAs and is regarded as less toxic, there would be less of a concern for human health.
148. The MOEs calculated for 97.5th percentile As exposures from drinking water for the UK are all greater than 10 and are considered to be of low concern for a risk to human health.
149. The MOE calculated for As exposure from the air is significantly greater than 10 and considered to be of low concern for a risk to human health.
150. The calculated large MOEs for soil exposure indicate that in the 75th percentile of measured As levels (assumed iAs), there is a low risk to human health, however, this is based upon ingestion rates of high uncertainty.
151. Toxicity will depend on total exposure to iAs from all sources, it is therefore important to consider this to determine an overall likely level of risk. The aggregate combined exposure from all sources using upper bound occurrence data and the 97.5th percentile of exposure from the diet (with mean exposure from all other sources) gives an MOE of 5.7 compared to the BMDL0.5 of 3.0 µg/kg bw/day. This conservative assessment of exposure could indicate that a risk for the health of women of childbearing age cannot be excluded.
152. When comparing the estimated exposures from different sources, it becomes apparent that dietary sources contribute more significantly to exposure than non-dietary sources such as water, air, soil, and dust and these exposures may represent a concern for health when estimated using upper bound occurrence data and 97.5th percentile consumption of food.
Questions for the Committee
1. Are there any further data the Committee would like to see added to the paper?
2. Do the Committee have any further thoughts or conclusions on the toxicity of organic arsenic species since they last reviewed this topic?
3. Does the Committee have any comments on how the risk characterisation has been undertaken for water, soil, dust, and air in terms of iAs being assumed?
4. Does the Committee have any additional comments on the structure or content of this discussion paper?
Secretariat
March 2023
Abbreviations
Table 10: List of abbreviations and their full meanings.
|
Abbreviation |
Meaning |
|
Acetyl-co A |
Acetyl coenzyme A |
|
ADME |
Absorption, Distribution, Metabolism and Elimination |
|
ADP |
Adenosine diphosphate |
|
AdoMet |
S-adenosyl-L-methionine |
|
ALARP |
As Low as Reasonably Practicable |
|
ArsM |
Methyltransferases |
|
AS3MT |
Arsenic methyltransferase |
|
ATP |
Adenosine triphosphate |
|
BKMR |
Bayesian kernel machine regression |
|
BMDL |
Benchmark dose level |
|
BMI |
Body mass index |
|
CAT |
Catylase |
|
CHD |
Congenital heart disease |
|
CLEA |
Environment Agency in their Contaminated Land Exposure Assessment |
|
COT |
Committee on Toxicity |
|
CONTAM |
The Panel on Contaminants in the Food Chain |
|
DDST |
Denver development screening test |
|
DMPS |
Diphenylmethylsilyl ether |
|
DTU |
Differential transcript usage |
|
E2 |
Estradiol |
|
EC |
European Commission |
|
EDTA |
Ethylenediaminetetraacetic acid |
|
EFSA |
The European Food Safety Authority |
|
ERK |
Extracellular signal-regulated kinase |
|
FDA |
Food and Drug Administration |
|
FSA |
Food Standards Agency |
|
GI |
Gastrointestinal |
|
GSH |
Glutathione |
|
GPx |
Glutathione peroxidase |
|
HDP |
Hypertensive Disorders of Pregnancy |
|
IgG |
Total immunoglobin |
|
IARC |
International Agency for Research |
|
IL2 |
Interleukin-2 |
|
JECFA |
The Joint FAO/WHO Expert Committee on Food Additives |
|
ln-unit |
Natural log unit increase |
|
ML |
Maximum Levels |
|
MN |
Micronucleus |
|
MOE |
Margin of Exposure |
|
mtDNAcn |
Mitochondrial DNA copy number |
|
NBNA |
Neonatal behavioural neurological assessment |
|
NDNS |
National Diet and Nutrition Survey |
|
NHS |
National Health Service |
|
OR4S2 |
Olfactory Receptor, Family 4, Subfamily S, Member 2 |
|
P4 |
Progesterone |
|
Papp |
Permeability coefficient value |
|
PGR |
Progesterone Receptor |
|
PLCB1 |
Phospholipase C, Beta 1 |
|
PMTDI |
Provisional Maximum Tolerable Daily Intake |
|
PMWI |
Provisional Tolerable Weekly Intake |
|
RNS |
Reactive Nitrogen Species |
|
ROS |
Reactive Oxygen Species |
|
RSC |
Royal Society of Chemistry |
|
SACN |
The Scientific Advisory Committee on Nutrition |
|
SAM |
S-adenosylmethionine |
|
SD |
Standard deviation |
|
SGA |
Small for Gestational Age |
|
T |
Testosterone |
|
TTP |
Time to pregnancy |
|
UK |
United Kingdom |
|
USEPA |
United States Environmental Protection Agency |
|
WHO |
World Health Organisation |
|
P97.5 |
97.5th confidence interval |
Search Terms
The references cited in this discussion paper are of publications found in Ebsco, Pubmed, Scopus and Springer, searched using Lit fetch. The publications retrieved were selected using the following search terms:
|
Arsenic Arsenolipids Arsenosugars Arsenobetaine Arsenocholine Organoarsenic |
AND
|
Pregnancy Preconception Lactation Fertility Pregnancy Chances Birth Outcomes Absorption Distribution Metabolism |
References
Ahmad, S.A. et al. (2001) ‘Arsenic in drinking water and pregnancy outcomes.’, Environmental Health Perspectives, 109(6), pp. 629–631.Available at: https://doi.org/10.1289/ehp.01109629.
Ahmed, S.M. et al. (2019) ‘A Prospective Cohort Study Examining the Associations of Maternal Arsenic Exposure With Fetal Loss and Neonatal Mortality’, American Journal of Epidemiology, 188(2), pp. 347–354. Available at: https://doi.org/10.1093/aje/kwy243.
Akram, Z. et al. (2010) ‘Adverse effects of arsenic exposure on uterine function and structure in female rat’, Experimental and Toxicologic Pathology, 62(4), pp. 451–459. Available at: https://doi.org/10.1016/j.etp.2009.07.008.
Almberg, K.S. et al. (2017) ‘Arsenic in drinking water and adverse birth outcomes in Ohio’, Environmental Research, 157, pp. 52–59. Available at: https://doi.org/10.1016/j.envres.2017.05.010.
Amayo, K.O. et al. (2013) ‘Novel Identification of Arsenolipids Using Chemical Derivatizations in Conjunction with RP-HPLC-ICPMS/ESMS’, Analytical Chemistry, 85(19), pp. 9321–9327. Available at: https://doi.org/10.1021/ac4020935.
Ander, E., MR Cave and Johnson, C. (2013) Normal background concentrations of contaminants in the soils of Wales, pp. 1–144. Available at: BGS Report, single column layout (nerc.ac.uk).
Ander, E.L., Johnson, M. and Palumbo-Roe, B. (2011) Normal background concentrations of contaminants in the soils of England., pp. 1–124. Available at: BGS Report, single column layout (nerc.ac.uk).
Andrews, F.V. et al. (2022) ‘A prospective study of arsenic and manganese exposures and maternal blood pressure during gestation’, Environmental Research, 214, p. 113845. Available at: https://doi.org/10.1016/j.envres.2022.113845.
de Assis Araujo, M.S. et al. (2022) ‘Prenatal Exposure to Metals and Neurodevelopment in Infants at Six Months: Rio Birth Cohort Study of Environmental Exposure and Childhood Development (PIPA Project)’, International Journal of Environmental Research and Public Health, 19(7), p. 4295. Available at: https://doi.org/10.3390/ijerph19074295.
Attreed, S.E., Navas-Acien, A. and Heaney, C.D. (2017) ‘Arsenic and Immune Response to Infection During Pregnancy and Early Life’, Current Environmental Health Reports, 4(2), pp. 229–243. Available at: https://doi.org/10.1007/s40572-017-0141-4.
Biggio, J. et al. (2015) ‘9: Neonatal, not maternal, copy number variants are associated with spontaneous preterm birth’, American Journal of Obstetrics and Gynecology, 212(1), p. S8. Available at: https://doi.org/10.1016/j.ajog.2014.10.055.
Bloom, M.S. et al. (2014) ‘Consumption of low-moderate level arsenic contaminated water does not increase spontaneous pregnancy loss: a case control study’, Environmental Health, 13(1), p. 81. Available at: https://doi.org/10.1186/1476-069X-13-81.
Bolan, S. et al. (2021) ‘Bioavailability of arsenic, cadmium, lead and mercury as measured by intestinal permeability’, Scientific Reports, 11(1), p. 14675. Available at: https://doi.org/10.1038/s41598-021-94174-9.
Bommarito, P.A. et al. (2019) ‘Urinary trace metals, maternal circulating angiogenic biomarkers, and preeclampsia: a single-contaminant and mixture-based approach’, Environmental Health, 18(1), p. 63. Available at: https://doi.org/10.1186/s12940-019-0503-5.
Bornhorst, J. et al. (2020) ‘Toxicity of three types of arsenolipids: species-specific effects in Caenorhabditis elegans’, Metallomics, 12(5), pp. 794–798. Available at: https://doi.org/10.1039/d0mt00039f.
Buchet, J.P., Lauwerys, R. and Roels, H. (1981) ‘Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man’, International Archives of Occupational and Environmental Health, 48(1), pp. 71–79. Available at: https://doi.org/10.1007/BF00405933.
Calatayud, M. et al. (2018) ‘Salivary and Gut Microbiomes Play a Significant Role in in Vitro Oral Bioaccessibility, Biotransformation, and Intestinal Absorption of Arsenic from Food’, Environmental Science & Technology, 52(24), pp. 14422–14435. Available at: https://doi.org/10.1021/acs.est.8b04457.
Cardenas, A. et al. (2015) ‘Arsenic Exposure and Prevalence of the Varicella Zoster Virus in the United States: NHANES (2003–2004 and 2009–2010)’, Environmental Health Perspectives, 123(6), pp. 590–596. Available at: https://doi.org/10.1289/ehp.1408731.
Challenger, F. (1955) ‘Biological methylation’, Quarterly Reviews, Chemical Society, 9(3), p. 255. Available at: https://doi.org/10.1039/qr9550900255.
Chandravanshi, L.P., Gupta, R. and Shukla, R.K. (2019) ‘Arsenic-Induced Neurotoxicity by Dysfunctioning Cholinergic and Dopaminergic System in Brain of Developing Rats’, Biological Trace Element Research, 189(1), pp. 118–133. Available at: https://doi.org/10.1007/s12011-018-1452-5.
Chatterjee, A. and Chatterji, U. (2010) ‘Arsenic abrogates the estrogen-signaling pathway in the rat uterus’, Reproductive Biology and Endocrinology, 8(1), p. 80. Available at: https://doi.org/10.1186/1477-7827-8-80.
Chávez-Capilla, T. (2022) ‘The Need to Unravel Arsenolipid Transformations in Humans’, DNA and Cell Biology, 41(1), pp. 64–70. Available at: https://doi.org/10.1089/dna.2021.0476.
Chen, C.-L. et al. (2010) ‘Ingested arsenic, characteristics of well water consumption and risk of different histological types of lung cancer in northeastern Taiwan’, Environmental Research, 110(5), pp. 455–462. Available at: https://doi.org/10.1016/j.envres.2009.08.010.
Chen, P. et al. (2022) ‘Arsenic exposure during juvenile and puberty significantly affected reproductive system development of female SD rats’, Ecotoxicology and Environmental Safety, 242, p. 113857. Available at: https://doi.org/10.1016/j.ecoenv.2022.113857.
Chen, Y. et al. (2011) ‘Association between arsenic exposure from drinking water and proteinuria: results from the Health Effects of Arsenic Longitudinal Study’, International Journal of Epidemiology, 40(3), pp. 828–835. Available at: https://doi.org/10.1093/ije/dyr022.
Cherry, N. (2008) ‘Stillbirth in rural Bangladesh: arsenic exposure and other etiological factors: a report from Gonoshasthaya Kendra’, Bulletin of the World Health Organization, 86(3), pp. 172–177. Available at: https://doi.org/10.2471/BLT.07.043083.
Clark, J. et al. (2022) ‘Maternal serum concentrations of one-carbon metabolism factors modify the association between biomarkers of arsenic methylation efficiency and birth weight’, Environmental Health, 21(1), p. 68. Available at: https://doi.org/10.1186/s12940-022-00875-7.
Cobo, JoséM. and Castiñeira, M. (1997) ‘Oxidative stress, mitochondrial respiration, and glycemic control: Clues from chronic supplementation with Cr3+ or As3+ to male wistar rats’, Nutrition, 13(11–12), pp. 965–970. Available at: https://doi.org/10.1016/S0899-9007(97)00338-9.
COC (2022) Cancer risk characterisation methods. Available at: Risk characterisation methods - GOV.UK (www.gov.uk) (Accessed: 25 January 2023).
Commission Regulation (EU) 2015/1006 (2015) OJ L. Available at: http://data.europa.eu/eli/reg/2015/1006/oj/eng (Accessed: 10 January 2023).
Concha, G. et al. (1998) ‘Exposure to Inorganic Arsenic Metabolites during Early Human Development’, Toxicological Sciences, 44(2), pp. 185–190. Available at: https://doi.org/10.1093/toxsci/44.2.185.
COT (2003) ‘Statement on arsenic in food: results of the 1999 total diet study’, pp. 1–11.
COT (2008) ‘COT Statement on the 2006 UK Total Diet Study of Metals and Other Elements’, pp. 1–38.
COT (2016) ‘Statement on potential risks from arsenic in the diet of infants aged 0 to 12 months and children aged 1 to 5 years’, pp. 1–38.
Cullen, W.R., McBride, B.C. and Pickett, A.W. (1979) ‘The transformation of arsenicals by Candida humicola’, Canadian Journal of Microbiology, 25(10), pp. 1201–1205. Available at: https://doi.org/10.1139/m79-187.
Dash, M. et al. (2018) ‘The consequence of NAC on sodium arsenite-induced uterine oxidative stress’, Toxicology Reports, 5, pp. 278–287. Available at: https://doi.org/10.1016/j.toxrep.2018.02.003.
Davey, J.C. et al. (2008) ‘Arsenic as an Endocrine Disruptor: Arsenic Disrupts Retinoic Acid Receptor–and Thyroid Hormone Receptor–Mediated Gene Regulation and Thyroid Hormone–Mediated Amphibian Tail Metamorphosis’, Environmental Health Perspectives, 116(2), pp. 165–172. Available at: https://doi.org/10.1289/ehp.10131.
Dávila-Esqueda, Ma.E. et al. (2012) ‘Effects of arsenic exposure during the pre- and postnatal development on the puberty of female offspring’, Experimental and Toxicologic Pathology, 64(1–2), pp. 25–30. Available at: https://doi.org/10.1016/j.etp.2010.06.001.
Davis, M.A. et al. (2015) ‘Preliminary analysis of in utero low-level arsenic exposure and fetal growth using biometric measurements extracted from fetal ultrasound reports’, Environmental Health, 14(1), p. 12. Available at: https://doi.org/10.1186/1476-069X-14-12.
Department of Environment Food Rural Affairs (2020) UK Air - Air Information Resource, UK Air - Air Information Resource. Available at: AQMAs interactive map (defra.gov.uk) (Accessed: 1 February 2023).
Devesa, V. et al. (2006) ‘Arsenicals in maternal and fetal mouse tissues after gestational exposure to arsenite’, Toxicology, 224(1–2), pp. 147–155. Available at: https://doi.org/10.1016/j.tox.2006.04.041.
Devick, K.L. et al. (2022) ‘Bayesian kernel machine regression‐causal mediation analysis’, Statistics in Medicine, 41(5), pp. 860–876. Available at: https://doi.org/10.1002/sim.9255.
Deyssenroth, M.A. et al. (2022) ‘Placental Gene Transcript Proportions are Altered in the Presence of In Utero Arsenic and Cadmium Exposures, Genetic Variants, and Birth Weight Differences’, Frontiers in Genetics, 13, p. 865449. Available at: https://doi.org/10.3389/fgene.2022.865449.
Dheeman, D.S. et al. (2014) ‘Pathway of Human AS3MT Arsenic Methylation’, Chemical Research in Toxicology, 27(11), pp. 1979–1989. Available at: https://doi.org/10.1021/tx500313k.
Ebert, F. et al. (2016) ‘Toxicological characterisation of a thio-arsenosugar-glycerol in human cells’, Journal of Trace Elements in Medicine and Biology, 38, pp. 150–156. Available at: https://doi.org/10.1016/j.jtemb.2016.04.013.
EFSA (2014) ‘Dietary exposure to inorganic arsenic in the European population’, pp. 1–68. Available at: https://doi.org/10.2903/j.efsa.2014.3597.
EFSA (2021) ‘Chronic dietary exposure to inorganic arsenic’, EFSA Journal, 19(1). Available at: https://doi.org/10.2903/j.efsa.2021.6380.
EFSA CONTAM (2009) ‘Scientific Opinion on Arsenic in Food’, EFSA Journal, 7(10), p. 1351. Available at: https://doi.org/10.2903/j.efsa.2009.1351.
von Ehrenstein, O.S. et al. (2006) ‘Pregnancy Outcomes, Infant Mortality, and Arsenic in Drinking Water in West Bengal, India’, American Journal of Epidemiology, 163(7), pp. 662–669. Available at: https://doi.org/10.1093/aje/kwj089.
Elshawarby, A.M. et al. (2014) ‘Arsenic-induced toxicity in the endometrium of adult albino rat and the possible role of human chorionic gonadotropin hormone: a histological study’, The Egyptian Journal of Histology, 37(2), pp. 327–338. Available at: https://doi.org/10.1097/01.EHX.0000446582.73701.1b.
Engström, K.S. et al. (2007) ‘Genetic Polymorphisms Influencing Arsenic Metabolism: Evidence from Argentina’, Environmental Health Perspectives, 115(4), pp. 599–605. Available at: https://doi.org/10.1289/ehp.9734.
Fängström, B. et al. (2008) ‘Breast-feeding Protects against Arsenic Exposure in Bangladeshi Infants’, Environmental Health Perspectives, 116(7), pp. 963–969. Available at: https://doi.org/10.1289/ehp.11094.
FAO/WHO (2011) ‘Safety evaluation of certain contaminants in food’, pp. 1–791.
Farzan, S.F. et al. (2013) ‘In utero arsenic exposure and infant infection in a United States cohort: A prospective study’, Environmental Research, 126, pp. 24–30. Available at: https://doi.org/10.1016/j.envres.2013.05.001.
Fawcett, E.J., Fawcett, J.M. and Mazmanian, D. (2016) ‘A meta-analysis of the worldwide prevalence of pica during pregnancy and the postpartum period’, International Journal of Gynecology & Obstetrics, 133(3), pp. 277–283. Available at: https://doi.org/10.1016/j.ijgo.2015.10.012.
FDA (2022) Arsenic in Food and Dietary Supplements, Arsenic in Food and Dietary Supplements. Available at: Arsenic in Food and Dietary Supplements | FDA (Accessed: 10 January 2023).
Fei, D.L. et al. (2013) ‘Association between In Utero arsenic exposure, placental gene expression, and infant birth weight: a US birth cohort study’, Environmental Health, 12(1), p. 58. Available at: https://doi.org/10.1186/1476-069X-12-58.
FERA (2015) ‘Total Diet Study of metals and other elements in food’, pp. 1–69.
Francesconi, K.A., Stick, R.V. and Edmonds, J.S. (1990) ‘Glycerylphosphorylarsenocholine and phosphatidylarsenocholine in yelloweye mullet (Aldrichetta forsteri) following oral administration of arsenocholine’, Experientia, 46(5), pp. 464–466. Available at: https://doi.org/10.1007/BF01954231.
FSA (2018) Arsenic in rice, Arsenic in Rice. Available at: Arsenic in rice | Food Standards Agency (Accessed: 10 January 2023).
Gao, S. et al. (2019) ‘Determinants of arsenic methylation efficiency and urinary arsenic level in pregnant women in Bangladesh’, Environmental Health, 18(1), p. 94. Available at: https://doi.org/10.1186/s12940-019-0530-2.
García-Salgado, S. et al. (2012) ‘Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae’, Environmental Chemistry, 9(1), p. 63. Available at: https://doi.org/10.1071/EN11164.
Gardner, R.M. et al. (2012) ‘Pregnancy and the methyltransferase genotype independently influence the arsenic methylation phenotype’, Pharmacogenetics and Genomics, 22(7), pp. 508–516. Available at: https://doi.org/10.1097/FPC.0b013e3283535d6a.
Gebel, T.W. (2002) ‘Arsenic methylation is a process of detoxification through accelerated excretion’, International Journal of Hygiene and Environmental Health, 205(6), pp. 505–508. Available at: https://doi.org/10.1078/1438-4639-00177.
Ghersevich, S. et al. (1994) ‘Rat 17 beta-hydroxysteroid dehydrogenase type 1: primary structure and regulation of enzyme expression in rat ovary by diethylstilbestrol and gonadotropins in vivo.’, Endocrinology, 135(4), pp. 1477–1487. Available at: https://doi.org/10.1210/endo.135.4.7925110.
Giri, B. and Dey, S. (2017) ‘Is it possible to avert arsenic effects on cells and tissues bypassing its toxicity and suppressive consequences of energy production? A hypothesis’, BLDE University Journal of Health Sciences, 2(2), p. 91. Available at: https://doi.org/10.4103/bjhs.bjhs_28_17.
Glabonjat, R.A. et al. (2017) ‘A 2-O-Methylriboside Unknown Outside the RNA World Contains Arsenic’, Angewandte Chemie International Edition, 56(39), pp. 11963–11965. Available at: https://doi.org/10.1002/anie.201706310.
González de Chávez Capilla, T. (2018) The metabolism of arsenic in humans : bioaccessibility in the gastrointestinal tract, diffusion across lipid membranes and biotransformations in liver cells. University of Canberra. Available at: https://doi.org/10.26191/A6VF-ZF34.
Govarts, E. et al. (2016) ‘Combined Effects of Prenatal Exposures to Environmental Chemicals on Birth Weight’, International Journal of Environmental Research and Public Health, 13(5), p. 495. Available at: https://doi.org/10.3390/ijerph13050495.
GOV.UK (2019) Arsenic: general information, Arsenic: general information. Available at: Arsenic: general information - GOV.UK (www.gov.uk) (Accessed: 10 January 2023).
Guan, H. et al. (2012) ‘Prenatal Exposure to Arsenic and Its Effects on Fetal Development in the General Population of Dalian’, Biological Trace Element Research, 149(1), pp. 10–15. Available at: https://doi.org/10.1007/s12011-012-9396-7.
Hall, M. et al. (2007) ‘Determinants of Arsenic Metabolism: Blood Arsenic Metabolites, Plasma Folate, Cobalamin, and Homocysteine Concentrations in Maternal–Newborn Pairs’, Environmental Health Perspectives, 115(10), pp. 1503–1509. Available at: https://doi.org/10.1289/ehp.9906.
Hamadani, J.D. et al. (2010) ‘Pre- and postnatal arsenic exposure and child development at 18 months of age: a cohort study in rural Bangladesh’, International Journal of Epidemiology, 39(5), pp. 1206–1216. Available at: https://doi.org/10.1093/ije/dyp369.
Heaney, C.D. et al. (2015) ‘Arsenic exposure and hepatitis E virus infection during pregnancy’, Environmental Research, 142, pp. 273–280. Available at: https://doi.org/10.1016/j.envres.2015.07.004.
Hopenhayn, C. et al. (2003) ‘Profile of urinary arsenic metabolites during pregnancy.’, Environmental Health Perspectives, 111(16), pp. 1888–1891. Available at: https://doi.org/10.1289/ehp.6254.
Hopenhayn-Rich, C. et al. (2000) ‘Chronic arsenic exposure and risk of infant mortality in two areas of Chile.’, Environmental Health Perspectives, 108(7), pp. 667–673. Available at: https://doi.org/10.1289/ehp.00108667.
Hsu, L.-I. et al. (2015) ‘Association of Environmental Arsenic Exposure, Genetic Polymorphisms of Susceptible Genes, and Skin Cancers in Taiwan’, BioMed Research International, 2015, pp. 1–10. Available at: https://doi.org/10.1155/2015/892579.
Huang, H. et al. (2018) ‘Investigation of association between environmental and socioeconomic factors and preterm birth in California’, Environment International, 121, pp. 1066–1078. Available at: https://doi.org/10.1016/j.envint.2018.07.027.
IARC (2018) ‘Arsenic and Arsenic Compounds’, pp. 1–54.
Ishfaq Ahmad, H. et al. (2021) ‘Reproductive Toxicity of Arsenic: What We Know and What We Need to Know?’, in T. Otsuki (ed.) Environmental Health. IntechOpen. Available at: https://doi.org/10.5772/intechopen.95379.
Iwai-Shimada, M. et al. (2019) ‘Exposure profile of mercury, lead, cadmium, arsenic, antimony, copper, selenium and zinc in maternal blood, cord blood and placenta: the Tohoku Study of Child Development in Japan’, Environmental Health and Preventive Medicine, 24(1), p. 35. Available at: https://doi.org/10.1186/s12199-019-0783-y.
Jana, K., Jana, S. and Samanta, P.K. (2006) ‘Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action’, Reproductive Biology and Endocrinology, 4(1), p. 9. Available at: https://doi.org/10.1186/1477-7827-4-9.
JECFA (1989) ‘Evaluation of certain food additives and contaminants’. Available at: Evaluation of certain food additives and contaminants : thirty-third report of the Joint FAO/WHO Expert Committee on Food Additives [meeting held in Geneva from 21 to 30 March 1988] (Accessed: 10 January 2023).
JECFA (1993) Toxicological evaluation of certain food additives and contaminants. World Health Organization. Available at: Toxicological evaluation of certain food additives and contaminants / prepared by the forty-first meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA) [meeting held in Geneva, from 9 to 18 February 1993] (Accessed: 10 January 2023).
JECFA (2011) Evaludation of Certain Contaminants in Food. WHO, pp. 1–115. Available at: https://apps.who.int/iris/handle/10665/44514.
Jeffries, J. and Martin, I. (2009) Updated technical background to the CLEA model. Environment Agency, pp. 1–166. Available at: Microsoft Word - 0901115 CLEA Report for publication.doc (publishing.service.gov.uk).
Kabir, T. et al. (2020) ‘Arsenic hampered embryonic development: An in vivo study using local Bangladeshi Danio rerio model’, Toxicology Reports, 7, pp. 155–161. Available at: https://doi.org/10.1016/j.toxrep.2019.12.009.
Kenyon, E.M. et al. (2008) ‘Tissue distribution and urinary excretion of inorganic arsenic and its methylated metabolites in C57BL6 mice following subchronic exposure to arsenate in drinking water’, Toxicology and Applied Pharmacology, 232(3), pp. 448–455. Available at: https://doi.org/10.1016/j.taap.2008.07.018.
Khairul, I. et al. (2017) ‘Metabolism, toxicity and anticancer activities of arsenic compounds’, Oncotarget, 8(14), pp. 23905–23926. Available at: https://doi.org/10.18632/oncotarget.14733.
Kile, M.L. et al. (2014) ‘A prospective cohort study of the association between drinking water arsenic exposure and self-reported maternal health symptoms during pregnancy in Bangladesh’, Environmental Health, 13(1), p. 29. Available at: https://doi.org/10.1186/1476-069X-13-29.
Kile, M.L. et al. (2015) ‘Estimating effects of arsenic exposure during pregnancy on perinatal outcomes in a Bangladeshi cohort’:, Epidemiology, p. 1. Available at: https://doi.org/10.1097/EDE.0000000000000416.
Kim, M. et al. (2014) ‘Arsenic Exposure in Drinking Water Alters the Dopamine System in the Brains of C57BL/6 Mice’, Biological Trace Element Research, 162(1–3), pp. 175–180. Available at: https://doi.org/10.1007/s12011-014-0145-y.
Kohlmeyer, U., Kuballa, J. and Jantzen, E. (2002) ‘Simultaneous separation of 17 inorganic and organic arsenic compounds in marine biota by means of high-performance liquid chromatography/inductively coupled plasma mass spectrometry’, Rapid Communications in Mass Spectrometry, 16(10), pp. 965–974. Available at: https://doi.org/10.1002/rcm.671.
Kubota, R. et al. (2005) ‘Placental transfer of arsenic to fetus of Dall’s porpoises (Phocoenoides dalli)’, Marine Pollution Bulletin, 51(8–12), pp. 845–849. Available at: https://doi.org/10.1016/j.marpolbul.2005.01.011.
Le, X.C., Cullen, W.R. and Reimer, K.J. (1994) ‘Human urinary arsenic excretion after one-time ingestion of seaweed, crab, and shrimp’, Clinical Chemistry, 40(4), pp. 617–624. Available at: https://doi.org/10.1093/clinchem/40.4.617.
Leffers, L. et al. (2013) ‘In vitro toxicological characterization of two arsenosugars and their metabolites’, Molecular Nutrition & Food Research, 57(7), pp. 1270–1282. Available at: https://doi.org/10.1002/mnfr.201200821.
Liang, C. et al. (2020) ‘Low levels of arsenic exposure during pregnancy and maternal and neonatal thyroid hormone parameters: The determinants for these associations’, Environment International, 145, p. 106114. Available at: https://doi.org/10.1016/j.envint.2020.106114.
Lindgren, A. et al. (1984) ‘Embryotoxicity of Arsenite and Arsenate: Distribution in Pregnant Mice and Monkeys and Effects on Embryonic Cells in Vitro’, Acta Pharmacologica et Toxicologica, 54(4), pp. 311–320. Available at: https://doi.org/10.1111/j.1600-0773.1984.tb01936.x.
Liu, H. et al. (2022) ‘The Relationship Between Preeclampsia and Arsenic Concentration in the Peripheral Blood’, Biological Trace Element Research, 200(9), pp. 3965–3974. Available at: https://doi.org/10.1007/s12011-021-02988-5.
Liu, Z. et al. (2002) ‘Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9’, Proceedings of the National Academy of Sciences, 99(9), pp. 6053–6058. Available at: https://doi.org/10.1073/pnas.092131899.
Lu, K. et al. (2013) ‘Gut Microbiome Perturbations Induced by Bacterial Infection Affect Arsenic Biotransformation’, Chemical Research in Toxicology, 26(12), pp. 1893–1903. Available at: https://doi.org/10.1021/tx4002868.
Luo, L. et al. (2018) ‘Association between arsenic metabolism gene polymorphisms and arsenic-induced skin lesions in individuals exposed to high-dose inorganic arsenic in northwest China’, Scientific Reports, 8(1), p. 413. Available at: https://doi.org/10.1038/s41598-017-18925-3.
Luvonga, C. et al. (2020) ‘Organoarsenicals in Seafood: Occurrence, Dietary Exposure, Toxicity, and Risk Assessment Considerations – A Review’, Journal of Agricultural and Food Chemistry, 68(4), pp. 943–960. Available at: https://doi.org/10.1021/acs.jafc.9b07532.
Ma, W. et al. (2003) ‘Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation’, Proceedings of the National Academy of Sciences, 100(5), pp. 2963–2968. Available at: https://doi.org/10.1073/pnas.0530162100.
Maduray, K. et al. (2017) ‘Elemental analysis of serum and hair from pre-eclamptic South African women’, Journal of Trace Elements in Medicine and Biology, 43, pp. 180–186. Available at: https://doi.org/10.1016/j.jtemb.2017.03.004.
Marchiset-Ferlay, N., Savanovitch, C. and Sauvant-Rochat, M.-P. (2012) ‘What is the best biomarker to assess arsenic exposure via drinking water?’, Environment International, 39(1), pp. 150–171. Available at: https://doi.org/10.1016/j.envint.2011.07.015.
Mehta, M. and Hundal, S.S. (2016) ‘Effect of sodium arsenite on reproductive organs of female Wistar rats’, Archives of Environmental & Occupational Health, 71(1), pp. 16–25. Available at: https://doi.org/10.1080/19338244.2014.927346.
Meyer, S. et al. (2014) ‘In vitro toxicological characterisation of three arsenic-containing hydrocarbons’, Metallomics, 6(5), pp. 1023–1033. Available at: https://doi.org/10.1039/C4MT00061G.
Meyer, S. et al. (2015) ‘In vitro toxicological characterisation of arsenic-containing fatty acids and three of their metabolites’, Toxicology Research, 4(5), pp. 1289–1296. Available at: https://doi.org/10.1039/C5TX00122F.
Milton, A. et al. (2017) ‘A Review of the Effects of Chronic Arsenic Exposure on Adverse Pregnancy Outcomes’, International Journal of Environmental Research and Public Health, 14(6), p. 556. Available at: https://doi.org/10.3390/ijerph14060556.
Milton, A.H. et al. (2005) ‘Chronic Arsenic Exposure and Adverse Pregnancy Outcomes in Bangladesh’, Epidemiology, 16(1), pp. 82–86.
Miranda, M. et al. (2019) ‘Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain’, Frontiers in Cellular Neuroscience, 13, p. 363. Available at: https://doi.org/10.3389/fncel.2019.00363.
Mochizuki, H. (2019) ‘Arsenic Neurotoxicity in Humans’, International Journal of Molecular Sciences, 20(14), p. 3418. Available at: https://doi.org/10.3390/ijms20143418.
Mónaco, N.M. et al. (2018) ‘Low arsenic concentrations impair memory in rat offpring exposed during pregnancy and lactation: Role of α7 nicotinic receptor, glutamate and oxidative stress’, NeuroToxicology, 67, pp. 37–45. Available at: https://doi.org/10.1016/j.neuro.2018.04.011.
Müller, S.M. et al. (2017) ‘Effects of arsenolipids on in vitro blood-brain barrier model’, Archives of Toxicology, 92(2), pp. 823–832. Available at: https://doi.org/10.1007/s00204-017-2085-8.
Müller, S.M. et al. (2018) ‘Arsenic-containing hydrocarbons disrupt a model in vitro blood-cerebrospinal fluid barrier’, Journal of Trace Elements in Medicine and Biology, 49, pp. 171–177. Available at: https://doi.org/10.1016/j.jtemb.2018.01.020.
Müller, S. M. et al. (2018) ‘Arsenic-containing hydrocarbons: effects on gene expression, epigenetics, and biotransformation in HepG2 cells’, Archives of Toxicology, 92(5), pp. 1751–1765. Available at: https://doi.org/10.1007/s00204-018-2194-z.
Myers, S.L. et al. (2010) ‘Maternal drinking water arsenic exposure and perinatal outcomes in Inner Mongolia, China’, Journal of Epidemiology & Community Health, 64(4), pp. 325–329. Available at: https://doi.org/10.1136/jech.2008.084392.
Nath Barbhuiya, S., Barhoi, D. and Giri, S. (2021) ‘Impact of Arsenic on Reproductive Health’, in T. Otsuki (ed.) Environmental Health. IntechOpen. Available at: https://doi.org/10.5772/intechopen.101141.
Navasumrit, P. et al. (2019) ‘Exposure to arsenic in utero is associated with various types of DNA damage and micronuclei in newborns: a birth cohort study’, Environmental Health, 18(1), p. 51. Available at: https://doi.org/10.1186/s12940-019-0481-7.
NHS (2022) Safe weaning, Better Health Start for Life. Available at: Page not found - NHS (www.nhs.uk) (Accessed: 10 January 2023).
Niehoff, A.-C. et al. (2016) ‘Imaging by Elemental and Molecular Mass Spectrometry Reveals the Uptake of an Arsenolipid in the Brain of Drosophila melanogaster’, Analytical Chemistry, 88(10), pp. 5258–5263. Available at: https://doi.org/10.1021/acs.analchem.6b00333.
Ohta, T., Sakurai, T. and Fujiwara, K. (2004) ‘Effects of arsenobetaine, a major organic arsenic compound in seafood, on the maturation and functions of human peripheral blood monocytes, macrophages and dendritic cells’, Applied Organometallic Chemistry, 18(9), pp. 431–437. Available at: https://doi.org/10.1002/aoc.690.
Ommati, M.M. et al. (2020) ‘The mechanisms of arsenic-induced ovotoxicity, ultrastructural alterations, and autophagic related paths: An enduring developmental study in folliculogenesis of mice’, Ecotoxicology and Environmental Safety, 204, p. 110973. Available at: https://doi.org/10.1016/j.ecoenv.2020.110973.
Opresko, D. (1992) Toxicity Profiles, The Risk Assessment Information System. Available at: The Risk Assessment Information System (ornl.gov) (Accessed: 12 January 2023).
Ozturk, M. et al. (2022) ‘Arsenic and Human Health: Genotoxicity, Epigenomic Effects, and Cancer Signaling’, Biological Trace Element Research, 200(3), pp. 988–1001. Available at: https://doi.org/10.1007/s12011-021-02719-w.
Packianathan, C., Kandavelu, P. and Rosen, B.P. (2018) ‘The Structure of an As(III) S-Adenosylmethionine Methyltransferase with 3-Coordinately Bound As(III) Depicts the First Step in Catalysis’, Biochemistry, 57(28), pp. 4083–4092. Available at: https://doi.org/10.1021/acs.biochem.8b00457.
Paul, S., Majumdar, S. and Giri, A.K. (2015) ‘Genetic susceptibility to arsenic-induced skin lesions and health effects: a review’, Genes and Environment, 37(1), p. 23. Available at: https://doi.org/10.1186/s41021-015-0023-7.
Public Health England (2014) National Diet and Nutrition Survey Results from Years 1, 2, 3 and 4 (combined) of the Rolling Programme (2008/2009 – 2011/2012). Public Health England, pp. 1–158. Available at: Main heading (publishing.service.gov.uk) (Accessed: 7 February 2023).
Public Health England (2016a) Arsenic Toxicological Overview, Arsenic Toxicological Overview. Available at: Compendium of Chemical Hazards (publishing.service.gov.uk) (Accessed: 10 January 2023).
Public Health England (2016b) National Diet and Nutrition Survey Results from Years 5 and 6 (combined) of the Rolling Programme (2012/2013 – 2013/2014). Public Health England, pp. 1–29. Available at: Main heading (publishing.service.gov.uk) (Accessed: 7 February 2023).
Public Health England (2018) Results from Years 7 and 8 (combined) of the Rolling Programme (2014/2015 to 2015/2016). Public Health England, pp. 1–29. Available at: National Diet and Nutrition Survey (publishing.service.gov.uk).
Public Health England (2020) National Diet and Nutrition Survey Rolling programme Years 9 to 11 (2016/2017 to 2018/2019). Public Health England, pp. 1–29. Available at: National Diet and Nutrition Survey (publishing.service.gov.uk) (Accessed: 7 February 2023).
Punshon, T. et al. (2015) ‘Placental arsenic concentrations in relation to both maternal and infant biomarkers of exposure in a US cohort’, Journal of Exposure Science & Environmental Epidemiology, 25(6), pp. 599–603. Available at: https://doi.org/10.1038/jes.2015.16.
Quansah, R. et al. (2015) ‘Association of Arsenic with Adverse Pregnancy Outcomes/Infant Mortality: A Systematic Review and Meta-Analysis’, Environmental Health Perspectives, 123(5), pp. 412–421. Available at: https://doi.org/10.1289/ehp.1307894.
R.Abdel Hameed, E. (2020) ‘Arsenic and Cadmium Levels in Maternal and Umbilical Cord Blood and their Associations with Birth Outcomes’, Biomedical and Pharmacology Journal, 13(1), pp. 61–69. Available at: https://doi.org/10.13005/bpj/1861.
Rahman, A. et al. (2010) ‘Arsenic Exposure and Risk of Spontaneous Abortion, Stillbirth, and Infant Mortality’, Epidemiology, 21(6), pp. 797–804. Available at: https://doi.org/10.1097/EDE.0b013e3181f56a0d.
Rahman, A. et al. (2011) ‘Arsenic Exposure in Pregnancy Increases the Risk of Lower Respiratory Tract Infection and Diarrhea during Infancy in Bangladesh’, Environmental Health Perspectives, 119(5), pp. 719–724. Available at: https://doi.org/10.1289/ehp.1002265.
Raml, R. et al. (2009) ‘Individual Variability in the Human Metabolism of an Arsenic-Containing Carbohydrate, 2′,3′-Dihydroxypropyl 5-deoxy-5-dimethylarsinoyl-β- d -riboside, a Naturally Occurring Arsenical in Seafood’, Chemical Research in Toxicology, 22(9), pp. 1534–1540. Available at: https://doi.org/10.1021/tx900158h.
Ratnaike, R.N. (2003) ‘Acute and chronic arsenic toxicity’, Postgraduate Medical Journal, 79(933), pp. 391–396. Available at: https://doi.org/10.1136/pmj.79.933.391.
Řezanka, T. et al. (2019) ‘Arsenolipids in the green alga Coccomyxa (Trebouxiophyceae, Chlorophyta)’, Phytochemistry, 164, pp. 243–251. Available at: https://doi.org/10.1016/j.phytochem.2019.05.002.
Richter, F. et al. (2022) ‘Maternal exposure to arsenic in drinking water and risk of congenital heart disease in the offspring’, Environment International, 160, p. 107051. Available at: https://doi.org/10.1016/j.envint.2021.107051.
Rumpler, A. et al. (2008) ‘Arsenic-Containing Long-Chain Fatty Acids in Cod-Liver Oil: A Result of Biosynthetic Infidelity?’, Angewandte Chemie International Edition, 47(14), pp. 2665–2667. Available at: https://doi.org/10.1002/anie.200705405.
SACN (2011) ‘The influence of maternal, fetal and child nutrition on the development of chronic disease in later life’, pp. 1–192.
SACN (2018) ‘Feeding in the First Year of Life’, pp. 1–271.
Saha, A. et al. (2013) ‘Vaccine specific immune response to an inactivated oral cholera vaccine and EPI vaccines in a high and low arsenic area in Bangladeshi children’, Vaccine, 31(4), pp. 647–652. Available at: https://doi.org/10.1016/j.vaccine.2012.11.049.
Sandoval-Carrillo, A. et al. (2016) ‘Arsenic exposure and risk of preeclampsia in a Mexican mestizo population’, BMC Pregnancy and Childbirth, 16(1), p. 153. Available at: https://doi.org/10.1186/s12884-016-0946-4.
Schmeisser, E. et al. (2006) ‘Arsenic Fatty Acids Are Human Urinary Metabolites of Arsenolipids Present in Cod Liver’, Angewandte Chemie International Edition, 45(1), pp. 150–154. Available at: https://doi.org/10.1002/anie.200502706.
Schuhmacher–Wolz, U. et al. (2009) ‘Oral exposure to inorganic arsenic: evaluation of its carcinogenic and non-carcinogenic effects’, Critical Reviews in Toxicology, 39(4), pp. 271–298. Available at: https://doi.org/10.1080/10408440802291505.
Ser, P.H. et al. (2015) ‘Arsenic exposure increases maternal but not cord serum IgG in Bangladesh: Maternal arsenic exposure affects IgG’, Pediatrics International, 57(1), pp. 119–125. Available at: https://doi.org/10.1111/ped.12396.
Shi, X. et al. (2015) ‘Geospatial association between adverse birth outcomes and arsenic in groundwater in New Hampshire, USA’, Environmental Geochemistry and Health, 37(2), pp. 333–351. Available at: https://doi.org/10.1007/s10653-014-9651-2.
Shih, Y.-H. et al. (2017) ‘Associations between prenatal arsenic exposure with adverse pregnancy outcome and child mortality’, Environmental Research, 158, pp. 456–461. Available at: https://doi.org/10.1016/j.envres.2017.07.004.
Sitras, V. et al. (2009) ‘Placental Gene Expression Profile in Intrauterine Growth Restriction Due to Placental Insufficiency’, Reproductive Sciences, 16(7), pp. 701–711. Available at: https://doi.org/10.1177/1933719109334256.
Smeester, L. et al. (2017) ‘Toxic metals in amniotic fluid and altered gene expression in cell-free fetal RNA’, Prenatal Diagnosis, 37(13), pp. 1364–1366. Available at: https://doi.org/10.1002/pd.5183.
Song, L. et al. (2020) ‘Exposure to arsenic during pregnancy and newborn mitochondrial DNA copy number: A birth cohort study in Wuhan, China’, Chemosphere, 243, p. 125335. Available at: https://doi.org/10.1016/j.chemosphere.2019.125335.
Stone, J. et al. (2021) ‘Exposure to toxic metals and per- and polyfluoroalkyl substances and the risk of preeclampsia and preterm birth in the United States: a review’, American Journal of Obstetrics & Gynecology MFM, 3(3), p. 100308. Available at: https://doi.org/10.1016/j.ajogmf.2021.100308.
Suhl, J. et al. (2022) ‘Pre-pregnancy exposure to arsenic in diet and non-cardiac birth defects’, Public Health Nutrition, pp. 1–13. Available at: https://doi.org/10.1017/S1368980022001318.
Susko, M.L. et al. (2017) ‘Low-level arsenic exposure via drinking water consumption and female fecundity - A preliminary investigation’, Environmental Research, 154, pp. 120–125. Available at: https://doi.org/10.1016/j.envres.2016.12.030.
Taleshi, M.S. et al. (2008) ‘Arsenic-containing hydrocarbons: natural compounds in oil from the fish capelin, Mallotus villosus’, Chemical Communications, (39), p. 4706. Available at: https://doi.org/10.1039/b808049f.
Tam, G.K.H. et al. (1979) ‘Metabolism of inorganic arsenic (74As) in humans following oral ingestion’, Toxicology and Applied Pharmacology, 50(2), pp. 319–322. Available at: https://doi.org/10.1016/0041-008X(79)90157-1.
Taylor, V. et al. (2017) ‘Human exposure to organic arsenic species from seafood’, Science of The Total Environment, 580, pp. 266–282. Available at: https://doi.org/10.1016/j.scitotenv.2016.12.113.
Tchounwou, P.B. et al. (2015) ‘Arsenic and Cancer’, in Handbook of Arsenic Toxicology. Elsevier, pp. 533–555. Available at: https://doi.org/10.1016/B978-0-12-418688-0.00023-X.
Tchounwou, P.B. et al. (2019) ‘State of the science review of the health effects of inorganic arsenic: Perspectives for future research’, Environmental Toxicology, 34(2), pp. 188–202. Available at: https://doi.org/10.1002/tox.22673.
The Royal Society of Chemistry (2023) Arsenic, Arsenic. Available at: Arsenic - Element information, properties and uses | Periodic Table (rsc.org) (Accessed: 10 January 2023).
Thomas, D.J. (2021) ‘Arsenic methylation – Lessons from three decades of research’, Toxicology, 457, p. 152800. Available at: https://doi.org/10.1016/j.tox.2021.152800.
Tofail, F. et al. (2009) ‘Effect of Arsenic Exposure during Pregnancy on Infant Development at 7 Months in Rural Matlab, Bangladesh’, Environmental Health Perspectives, 117(2), pp. 288–293. Available at: https://doi.org/10.1289/ehp.11670.
Tolins, M., Ruchirawat, M. and Landrigan, P. (2014) ‘The Developmental Neurotoxicity of Arsenic: Cognitive and Behavioral Consequences of Early Life Exposure’, Annals of Global Health, 80(4), p. 303. Available at: https://doi.org/10.1016/j.aogh.2014.09.005.
United States, Agency for Toxic Substances and Disease Registry. Syracuse Research Corporation (2007) Toxicological profile for arsenic. United States, Agency for Toxic Substances and Disease Registry. Available at: https://doi.org/10.15620/cdc:11481.
US EPA (1997) Exposure Factors Handbook, pp. 1–1216. Available at: Error | Risk Assessment Portal | US EPA.
Vahter, M. (1999) ‘Methylation of Inorganic Arsenic in Different Mammalian Species and Population Groups’, Science Progress, 82(1), pp. 69–88. Available at: https://doi.org/10.1177/003685049908200104.
Vahter, M. (2002) ‘Mechanisms of arsenic biotransformation’, Toxicology, 181–182, pp. 211–217. Available at: https://doi.org/10.1016/S0300-483X(02)00285-8.
Viczek, S.A., Jensen, K.B. and Francesconi, K.A. (2016) ‘Arsenic-Containing Phosphatidylcholines: A New Group of Arsenolipids Discovered in Herring Caviar’, Angewandte Chemie International Edition, 55(17), pp. 5259–5262. Available at: https://doi.org/10.1002/anie.201512031.
Villa-Bellosta, R. and Sorribas, V. (2008) ‘Role of rat sodium/phosphate cotransporters in the cell membrane transport of arsenate’, Toxicology and Applied Pharmacology, 232(1), pp. 125–134. Available at: https://doi.org/10.1016/j.taap.2008.05.026.
Wang, H. et al. (2018) ‘Maternal serum arsenic level during pregnancy is positively associated with adverse pregnant outcomes in a Chinese population’, Toxicology and Applied Pharmacology, 356, pp. 114–119. Available at: https://doi.org/10.1016/j.taap.2018.07.030.
Wang, X. et al. (2021) ‘Arsenic exposure and metabolism in relation to blood pressure changes in pregnant women’, Ecotoxicology and Environmental Safety, 222, p. 112527. Available at: https://doi.org/10.1016/j.ecoenv.2021.112527.
Wang, X.-N. et al. (2017) ‘Protective Effects of Curcumin against Sodium Arsenite-induced Ovarian Oxidative Injury in a Mouse Model’, Chinese Medical Journal, 130(9), pp. 1026–1032. Available at: https://doi.org/10.4103/0366-6999.204927.
Wang, Y. et al. (2020) ‘Exposure to multiple metals and prevalence for preeclampsia in Taiyuan, China’, Environment International, 145, p. 106098. Available at: https://doi.org/10.1016/j.envint.2020.106098.
WHO (2000) ‘Air quality guidelines for Europe’, pp. 1–287.
WHO (2022) ‘Guidelines for drinking-water quality’, pp. 1–583.
WHO (2023) Arsenic, Arsenic. Available at: Arsenic (who.int) (Accessed: 10 January 2023).
Willhite, C.C. and Ferm, V.H. (1984) ‘Prenatal and Developmental Toxicology of Arsenicals’, in M. Friedman (ed.) Nutritional and Toxicological Aspects of Food Safety. Boston, MA: Springer US (Advances in Experimental Medicine and Biology), pp. 205–228. Available at: https://doi.org/10.1007/978-1-4684-4790-3_9.
Winterbottom, E.F. et al. (2019) ‘Transcriptome-wide analysis of changes in the fetal placenta associated with prenatal arsenic exposure in the New Hampshire Birth Cohort Study’, Environmental Health, 18(1), p. 100. Available at: https://doi.org/10.1186/s12940-019-0535-x.
Witt, B., Ebert, F., et al. (2017) ‘Assessing neurodevelopmental effects of arsenolipids in pre-differentiated human neurons’, Molecular Nutrition & Food Research, 61(11), p. 1700199. Available at: https://doi.org/10.1002/mnfr.201700199.
Witt, B., Meyer, S., et al. (2017) ‘Toxicity of two classes of arsenolipids and their water-soluble metabolites in human differentiated neurons’, Archives of Toxicology, 91(9), pp. 3121–3134. Available at: https://doi.org/10.1007/s00204-017-1933-x.
Xiong, C. et al. (2022) ‘Gut microbiota metabolize arsenolipids in a donor dependent way’, Ecotoxicology and Environmental Safety, 239, p. 113662. Available at: https://doi.org/10.1016/j.ecoenv.2022.113662.
Xu, L. et al. (2011) ‘Decrease in birth weight and gestational age by arsenic among the newborn in Shanghai, China’, [Nihon Koshu Eisei Zasshi] Japanese Journal of Public Health, 58(2), pp. 89–95.
Xue, X.-M. et al. (2017) ‘Arsenic Methyltransferase is Involved in Arsenosugar Biosynthesis by Providing DMA’, Environmental Science & Technology, 51(3), pp. 1224–1230. Available at: https://doi.org/10.1021/acs.est.6b04952.
Xue, X.M. et al. (2021) ‘The enigma of environmental organoarsenicals’, Critical Reviews in Environmental Science and Technology, 52, pp. 1–28. Available at: https://doi.org/10.1080/10643389.2021.1947678.
Yamamura, Y. and Yamauchi, H. (1980) ‘Arsenic metabolites in hair, blood and urine in workers exposed to arsenic trioxide.’, INDUSTRIAL HEALTH, 18(4), pp. 203–210. Available at: https://doi.org/10.2486/indhealth.18.203.
Yamauchi, H. and Yamamura, Y. (1983) ‘Concentration and chemical species of arsenic in human tissue’, Bulletin of Environmental Contamination and Toxicology, 31(3), pp. 267–270. Available at: https://doi.org/10.1007/BF01608697.
Yang, H.-C. et al. (2012) ‘Pathways of Arsenic Uptake and Efflux’, in Current Topics in Membranes. Elsevier, pp. 325–358. Available at: https://doi.org/10.1016/B978-0-12-394390-3.00012-4.
Yin, N. et al. (2016) ‘Variability of arsenic bioaccessibility and metabolism in soils by human gut microbiota using different in vitro methods combined with SHIME’, Science of The Total Environment, 566–567, pp. 1670–1677. Available at: https://doi.org/10.1016/j.scitotenv.2016.06.071.
Yin, N. et al. (2017) ‘Interindividual variability of soil arsenic metabolism by human gut microbiota using SHIME model’, Chemosphere, 184, pp. 460–466. Available at: https://doi.org/10.1016/j.chemosphere.2017.06.018.
Zargari, F. et al. (2022) ‘Arsenic, Oxidative Stress and Reproductive System’, Journal of Xenobiotics, 12(3), pp. 214–222. Available at: https://doi.org/10.3390/jox12030016.
Zaw, Y.H. and Taneepanichskul, N. (2019) ‘Blood heavy metals and brain-derived neurotrophic factor in the first trimester of pregnancy among migrant workers’, PLOS ONE. Edited by K. Hashimoto, 14(6), p. e0218409. Available at: https://doi.org/10.1371/journal.pone.0218409.
Zheng, Y. et al. (2021) ‘Effect of arsenic-containing hydrocarbon on the long-term potentiation at Schaffer Collateral-CA1 synapses from infantile male rat’, NeuroToxicology, 84, pp. 198–207. Available at: https://doi.org/10.1016/j.neuro.2021.04.002.
Appendices
Appendix 1: Names and Abbreviations for Arsenic Species (EFSA CONTAM, 2009)
Table 11: List of names, abbreviations, and comments for different As species.
|
Name |
Abbreviation |
Comment |
|
Inorganic arsenic |
iAs |
The total sum of As(III) and As(V). |
|
Arsenite |
As(III) |
Highly toxic compound found at low levels in most foods. |
|
Arsenate |
As(V) |
Highly toxic compound that is found at trace to low levels in foods. |
|
Arsenobetaine |
AB |
Non-toxic arsenic species abundant in most seafoods. |
|
Arsenocholine |
AC |
Found in seafood at trace levels. This species of As is readily oxidised to AB in biological systems. |
|
Arsenosugars |
N/A |
Abundant arsenic species found in seafoods. |
|
Arsenolipids |
N/A |
Arsenic species found in fatty fish and fish oils. |
|
Arsenic containing fatty acids |
AsFA |
Group of fat soluble arsenic species present in fish and seafood. |
|
Arsenic containing hydrocarbons |
AsHC |
One of the groups of arsenolipids. |
|
Methylarsonate |
MA(V) |
A metabolite of As found in human urine. Found in trace levels in seafood and terrestrial foods. |
|
Methylarsonite |
MA(III) |
A toxic metabolite of iAs found in human urine. Species not normally detected in foods. |
|
Methylarsenate |
MA |
N/A |
|
Dimethylarsinate |
DMA(V) |
Minor arsenic species in seafoods and some terrestrial foods; the major human urine metabolite of iAs, arsenosugars and arsenolipids. |
|
Dimethylarsinite |
DMA(III) |
An unstable, reactive metabolite of iAs found in human urine. Species not normally detected in foods as difficult to measure due to its instability. Species is highly toxic. |
|
Dimethylmonoarsenate |
DMA |
N/A |
|
Methylarsonous acid |
MMA(III) |
N/A |
|
Methylarsonic acid |
MAA(V) |
N/A |
|
Dimethylarsinous acid |
DMAA(III) |
N/A |
|
Dimethylarsinic acid |
DMAA(V) |
N/A |
|
Dimethylarsonic acid |
DMMAA(III) |
N/A |
|
Trimethylarsine oxide |
TMAO |
Arsenic species found in seafood. |
|
Tetramethylarsonium ion |
TETRA |
Arsenic species found in seafood. |
|
Trimethylarsonio propionate |
TMAP |
Arsenic species present in seafoods. |
|
Thio-dimethylarsinate |
Thio-DMA |
A metabolite of iAs and arsenosugars found in human urine. |
Appendix 2: Pathways of Arsenic Metabolism* (Thomas, 2021)
*Upper – conversion via challenger pathway. Lower – conversion via alternative pathway.
Appendix 3: Dietary Exposure from iAs and tAs using the TDS
Table 12: Estimated iAs population based exposure from food consumed by women of childbearing age using data obtained from the TDS.
|
Food Groups |
Mean iAs Exposure – LB to UB (µg/kg/day)* for Females 16-49 years |
P97.5 iAs Exposure – LB to UB (µg/kg/day)* for Females 16-49 years |
|
Bread |
0-0.012 |
0-0.031 |
|
Misc. Cereals |
0.027 |
0.076 |
|
Carcass meat |
0-0.0041 |
0-0.019 |
|
Offal |
0-0.00015 |
0-0.0026 |
|
Meat products |
0-0.0064 |
0-0.027 |
|
Poultry |
0-0.0093 |
0-0.033 |
|
Fish and seafood |
0-0.0044 |
0-0.021 |
|
Fats and oils |
0-0.0021 |
0-0.0064 |
|
Eggs |
0-0.0034 |
0-0.016 |
|
Sugars and confectionary |
0-0.0040 |
0-0.016 |
|
Green vegetables |
0-0.0067 |
0-0.028 |
|
Potatoes |
0.016 |
0.048 |
|
Other vegetables |
0-0.016 |
0-0.052 |
|
Canned vegetables |
0-0.0063 |
0-0.029 |
|
Fresh fruit |
0-0.014 |
0-0.054 |
|
Fruit products |
0-0.0092 |
0-0.051 |
|
Non-alcoholic beverages |
0-0.062 |
0-0.15 |
|
Milk |
0-0.0054 |
0-0.020 |
|
Dairy products |
0-0.0096 |
0-0.036 |
|
Nuts and seeds |
0-0.0010 |
0-0.0088 |
|
Alcoholic beverages |
0-0.005 |
0-0.033 |
|
Meat alternatives |
0-0.00057 |
0-0.0071 |
|
Snacks |
0-0.0013 |
0-0.006 |
|
Desserts |
0-0.0024 |
0-0.015 |
|
Condiments |
0-0.0060 |
0-0.023 |
|
Tap water only |
0-0.021 |
0-0.092 |
|
Bottled water still or carbonated |
0-0.0051 |
0-0.041 |
|
Total^ |
0.043-0.26 |
0.098-0.51 |
*Values have been rounded to two significant figures.
LB - Lower bound; UB - Upper bound.
^P97.5th total values were determined from a distribution of consumption of any combination of food / drink categories rather than by summation of the individual 97.5th percentiles values for each category.
Table 13: Estimated population based tAs exposure from food consumed by women of childbearing age using data obtained from the TDS (FERA, 2015).
|
Food Groups |
Mean tAs Exposure – LB to UB (µg/kg bw/day)* for Females 16-49 years |
P97.5 tAs Exposure – LB to UB (µg/kg bw/day)* for Females 16-49 years |
|
Bread |
0-0.006 |
0-0.015 |
|
Misc. Cereals |
0.018 |
0.053 |
|
Carcass meat |
0-0.0021 |
0-0.0094 |
|
Offal |
0-0.000077 |
0-0.0013 |
|
Meat products |
0-0.0016 |
0-0.0069 |
|
Poultry |
0.0062 |
0.022 |
|
Fish and seafood |
0.74 |
3.6 |
|
Fats and oils |
0-0.00052 |
0-0.0016 |
|
Eggs |
0-0.00085 |
0-0.0040 |
|
Sugars and confectionary |
0-0.001 |
0-0.004 |
|
Green vegetables |
0-0.0017 |
0-0.0069 |
|
Potatoes |
0-0.0066 |
0-0.019 |
|
Other vegetables |
0-0.0081 |
0-0.026 |
|
Canned vegetables |
0-0.0016 |
0-0.0073 |
|
Fresh fruit |
0-0.0070 |
0-0.027 |
|
Fruit products |
0-0.0023 |
0-0.013 |
|
Non-alcoholic beverages |
0-0.062 |
0-0.15 |
|
Milk |
0-0.0054 |
0-0.02 |
|
Dairy products |
0-0.0024 |
0-0.0090 |
|
Nuts and seeds |
0.00043 |
0.0037 |
|
Alcoholic beverages |
0-0.005 |
0-0.033 |
|
Meat alternatives |
0.00019 |
0.0024 |
|
Snacks |
0.0012 |
0.0055 |
|
Desserts |
0.00059 |
0.0038 |
|
Condiments |
0.0055 |
0.021 |
|
Tap water only |
0-0.0084 |
0-0.037 |
|
Bottled water still or carbonated |
0-0.0020 |
0-0.017 |
|
Total^ |
0.77-0.89 |
3.6-3.8 |
*Values have been rounded to two significant figures.
LB - Lower bound; UB - Upper bound.
^P97.5th total values were determined from a distribution of consumption of any combination of food / drink categories rather than by summation of the individual 97.5th percentiles values for each category.